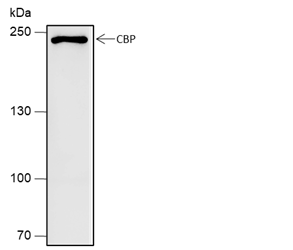
Background
CREB-binding protein (CBP) and p300 are highly conserved and functionally related transcriptional co-activators that associate with transcriptional regulators and signaling molecules, integrating multiple signal transduction pathways with the transcriptional machinery. CBP/p300 also contain histone acetyltransferase (HAT) activity, allowing them to acetylate histones and other proteins. Phosphorylation of p300 at Ser89 by PKC represses its transcriptional activity, and phosphorylation at the same site by AMPK disrupts the association of p300 with nuclear receptors. Ser1834 phosphorylation of p300 by Akt disrupts its association with C/EBPβ. Growth factors induce phosphorylation of CBP at Ser437, which is required for CBP recruitment to the transcription complex. CaM kinase IV phosphorylates CBP at Ser302, which is required for CBP-dependent transcriptional activation in the CNS. The role of acetylation of CBP/p300 is of particular interest. Acetylation of p300 at Lys1499 has been demonstrated to enhance its HAT activity and affect a wide variety of signaling events.
Cellular location
Cytoplasm, Nucleus