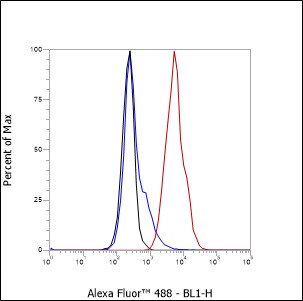
Background
Component of several heterodimeric amino acid transporter complexes. The precise substrate specificity depends on the other subunit in the heterodimer. The heterodimer with SLC3A2 functions as sodium-independent, high-affinity transporter that mediates uptake of large neutral amino acids such as phenylalanine, tyrosine, L-DOPA, leucine, histidine, methionine and tryptophan. The complexes with SLC7A6 and SLC7A7 mediate uptake of dibasic amino acids. The complexes function as amino acid exchangers. Required for targeting of SLC7A5 and SLC7A8 to the plasma membrane and for channel activity. Plays a role in nitric oxide synthesis in human umbilical vein endothelial cells (HUVECs) via transport of L-arginine. The heterodimer with SLC7A5/LAT1 may play a role in the transport of L-DOPA across the blood-brain barrier.
Cellular location
Membrane, Lysosome