Background
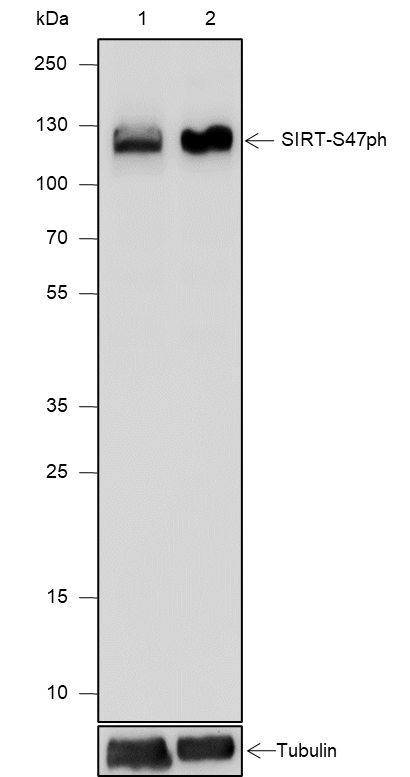
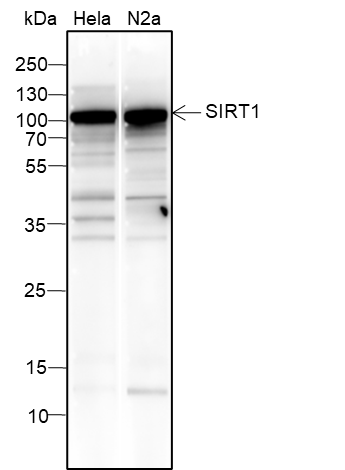
SIRT1, the mammalian ortholog of Sir2, is a nuclear protein implicated in the regulation of many cellular processes, including apoptosis, cellular senescence, endocrine signaling, glucose homeostasis, aging, and longevity. Targets of SirT1 include acetylated p53, p300, Ku70, forkhead (FoxO) transcription factors, PPARγ, and the PPARγ coactivator-1α (PGC-1α) protein. Deacetylation of p53 and FoxO transcription factors represses apoptosis and increases cell survival. Deacetylation of PPARγ and PGC-1α regulates the gluconeogenic/glycolytic pathways in the liver and fat mobilization in white adipocytes in response to fasting. SirT1 deacetylase activity is inhibited by nicotinamide and activated by resveratrol. In addition, SirT1 activity may be regulated by phosphorylation, as it is phosphorylated at Ser27 and Ser47 in vivo; however, the function of these phosphorylation sites has not yet been determined.
Cellular location
Cytoplasm, Nucleus