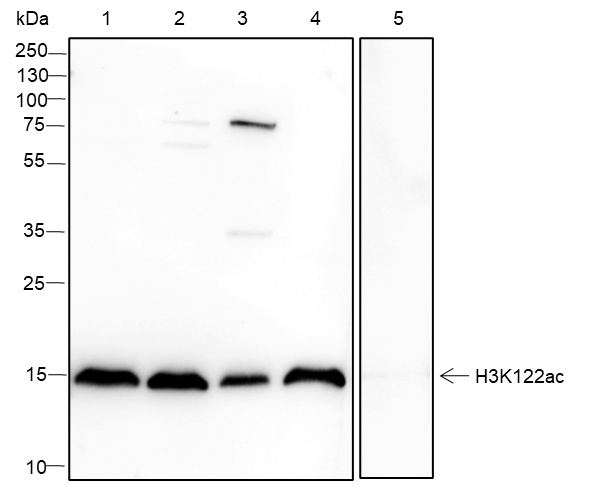
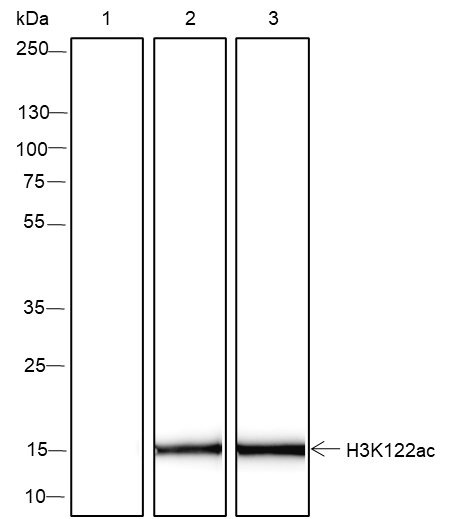
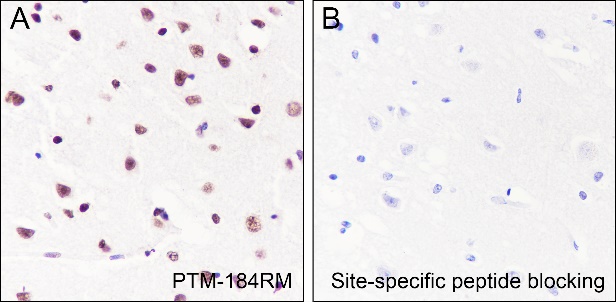
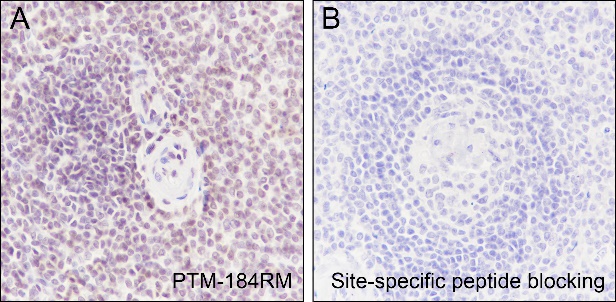
Background
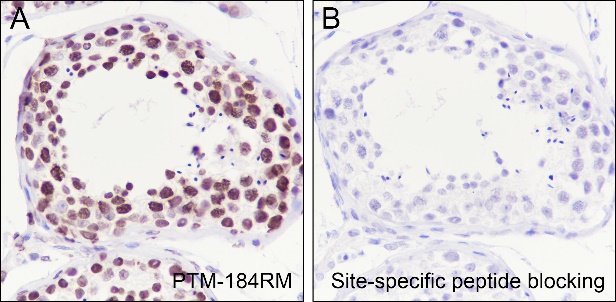
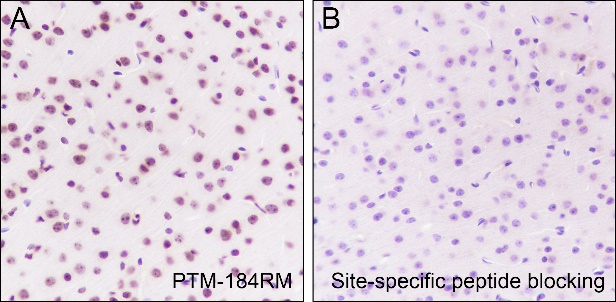
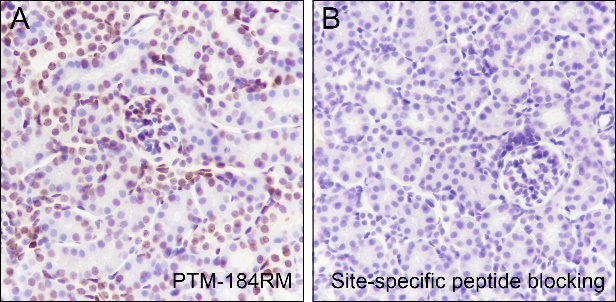
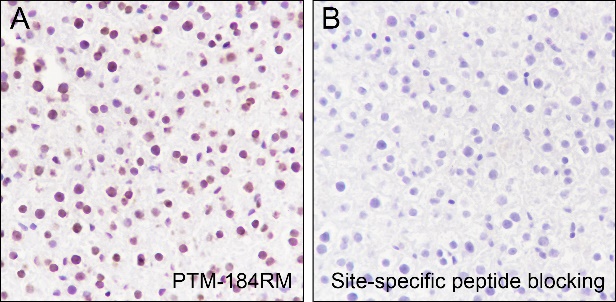
Histone post-translational modifications (PTMs), often conceptualized as the “histone code,” represent a key epigenetic mechanism for modulating chromatin structure, whereby diverse modifications including acetylation, methylation, phosphorylation, and novel acylations directly influence chromatin accessibility to regulatory factors, thereby altering genome stability and gene transcription. The specific modification of histone acetylation, which is dynamically regulated by the antagonistic activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs), is not restricted to the well-characterized N-terminal histone tails but also occurs within the structured globular domains. A prime example of this is Histone H3 Lysine 122 (H3K122), a highly conserved residue positioned at a vital interface near the DNA dyad axis. Acetylation at this specific site (H3K122ac) is a strong hallmark of active chromatin. It is highly enriched at the promoters and enhancers of actively transcribed genes, signifying its critical role in transcriptional activation.
Cellular location
Nucleus