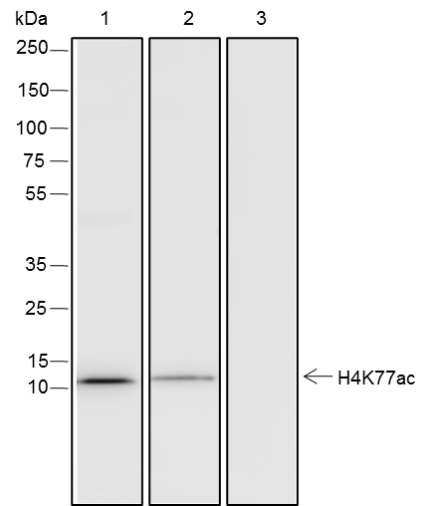
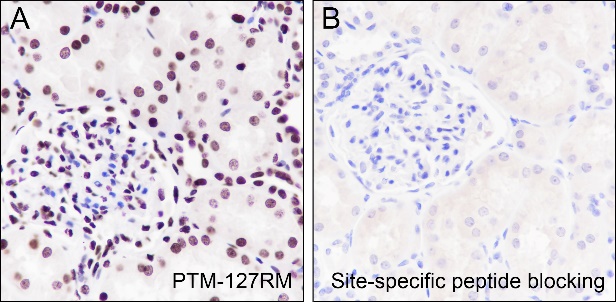
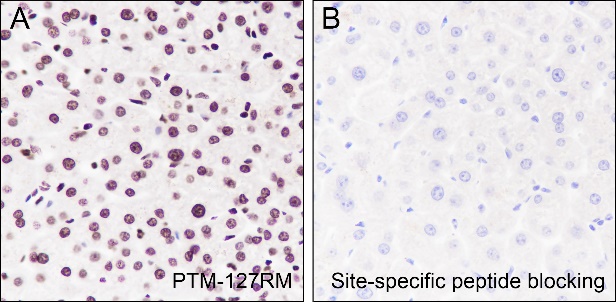
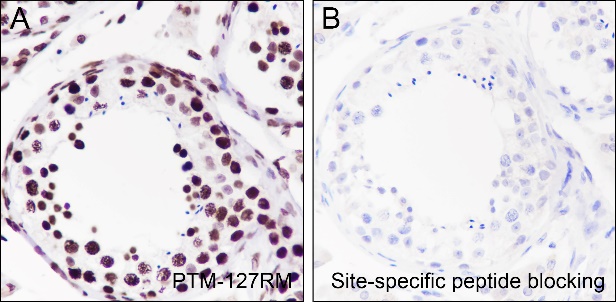
Background
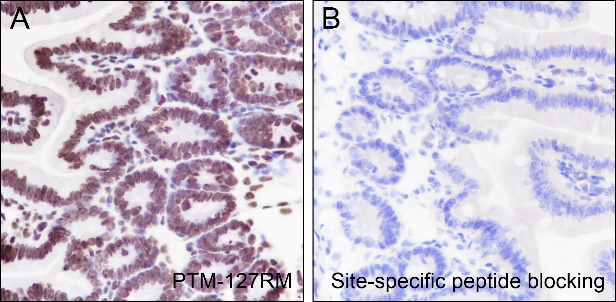
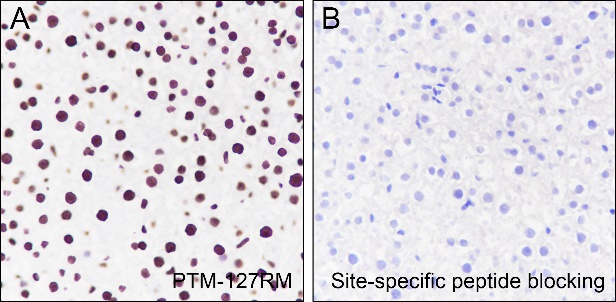
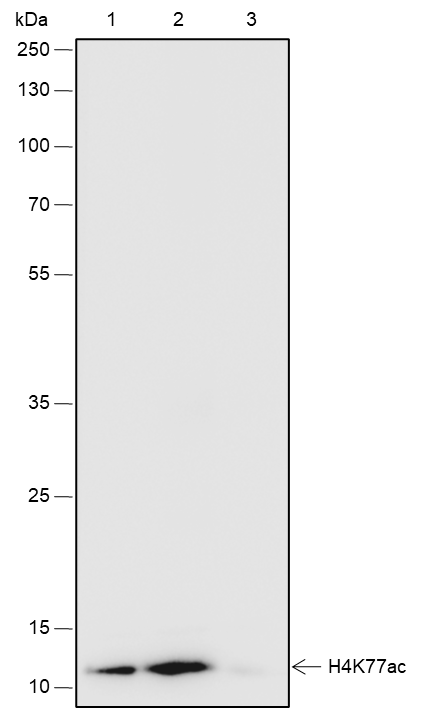
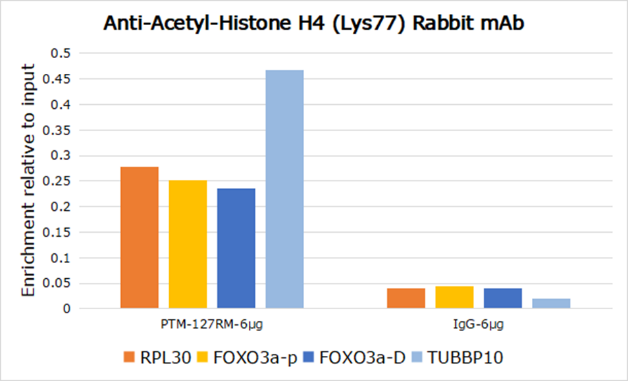
Histone post-translational modifications (PTMs), known as the “histone code”, are key mechanisms of epigenetics that modulate chromatin structures. The PTMs on histone including acetylation, methylation, phosphorylation, and novel acylations directly affect the accessibility of chromatin to transcription factors and other epigenetic regulators, altering genome stability and gene transcription. Histone acetylation, tightly controlled by the opposing action of histone acetyltransferases (HATs) and histone deacetylases (HDACs), occurs primarily at lysine residues on the N-terminal tails of histones H2A (Lys5, 9, and 15), H2B (Lys5, 12, 15, 16, and 20), H3 (Lys4, 9, 14, 18, 23, 27, and 36), and H4 (Lys5, 8, 12, 16, and 20), and plays vital roles in the regulation of gene expression, DNA damage repair, and chromatin dynamics. Recent research has identified that H2BK120ac, H3.3K18ac, and H4K77ac are significantly associated with the survival of hepatocellular carcinoma (HCC) patients. Furthermore, H4K77ac has been linked to HCC recurrence. These findings suggest that H2BK120ac, H3.3K18ac, and H4K77ac may be potential prognostic factors for HCC.
Cellular location
Nucleus