Background
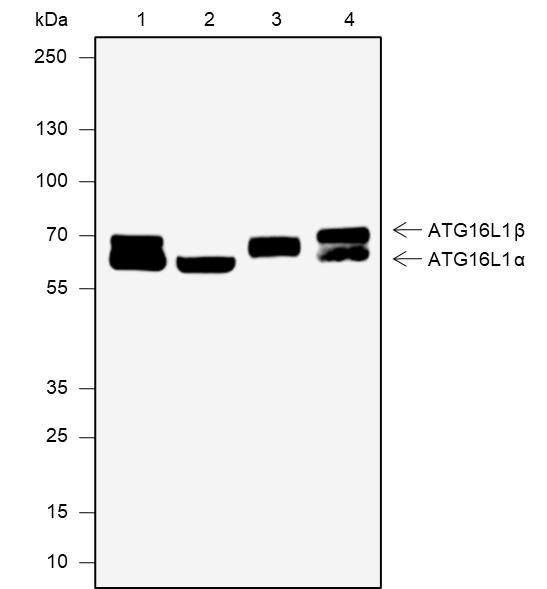
Atg16L1, containing an amino-terminal coiled-coil domain and carboxyl-terminal WD-repeats, has multiple isoforms produced by alternative splicing. Atg16L1 provides a functional link between the two crucial ubiquitin-like conjugation systems of autophagy. Atg16L1 binds Atg5 of the Atg12-Atg5 conjugate forming an 800 kDa multimeric complex. The Atg12-Atg-5-Atg16L1 complex localizes to pre-autophagosomal membranes where it determines the site of LC3 lipidation and catalyzes the reaction required for the formation of mature autophagosomes. Genome-wide association scanning revealed variations in the Atg16L1 gene associated with Crohn's disease. Mice lacking the coiled-coil domain of Atg16L1 have impaired autophagosome formation and elevated inflammatory cytokines, consistent with its role in inflammatory disease pathogenesis. Hypomorphic Atg16L1 mice also show defects in autophagy and abnormalities in intestinal Paneth cell function similar to that found in Crohn's disease.
Cellular location
Cytoplasm, Membrane