Background
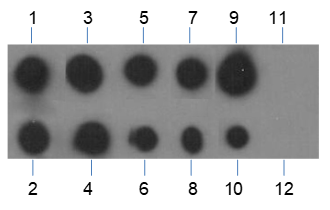
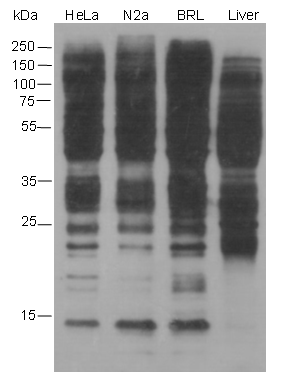
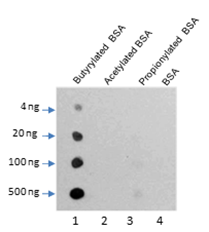
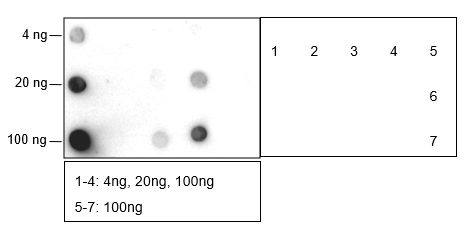
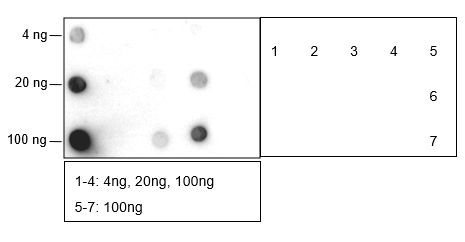
Butyrylation of lysine, structurally similar to lysine acetylation and lysine propionylation, is a newly identified reversible modification controlling protein activity. With integrated proteomic approaches and biochemistry analysis, lysine butyrylation has been well demonstrated in both prokaryotes and eukaryotes in wide ranges of proteins including histones and non-histone substrates. Given the facts that many lysine residues in histones and non-histone substrates, such as p53, p300/CBP, are butyrylated, lysine butyrylation may play a vital role in epigenetic modulation by impacting on chromatin dynamics and plasticity, DNA transcriptional regulation and tumorigenesis, etc.
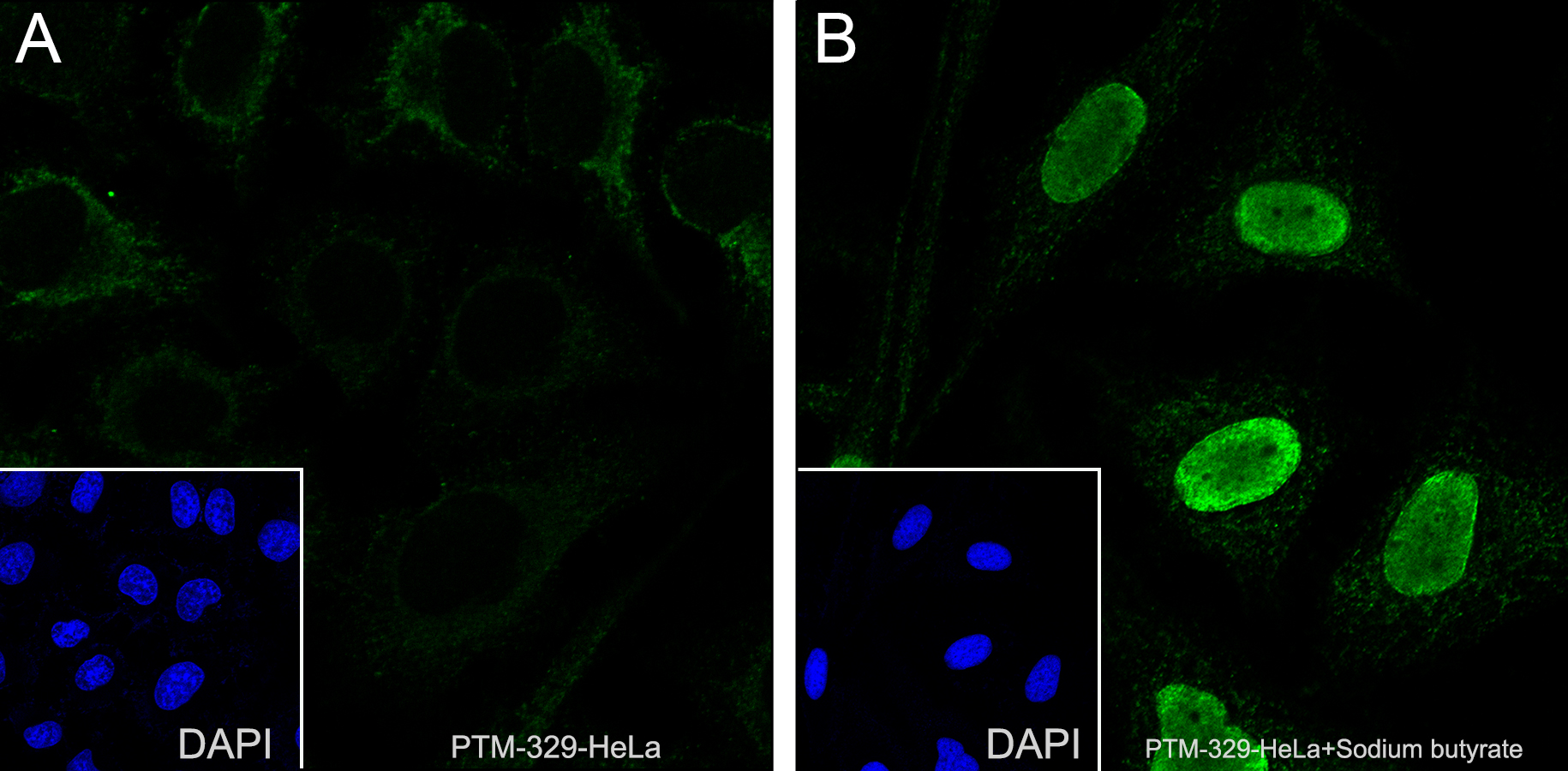
Cellular location
/