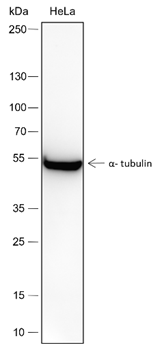
Background
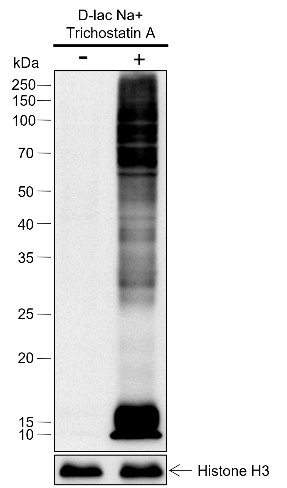
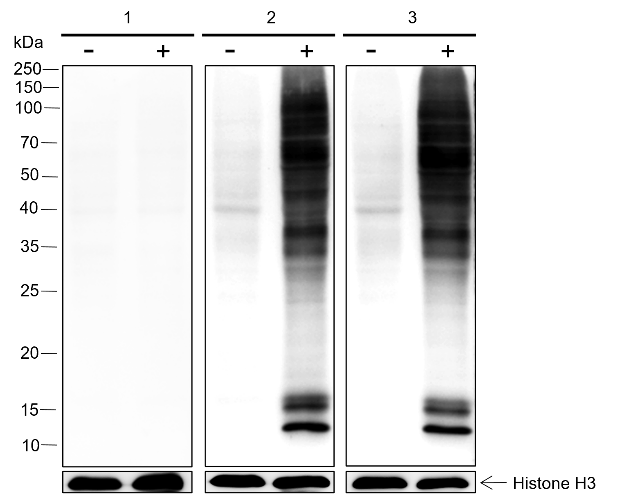
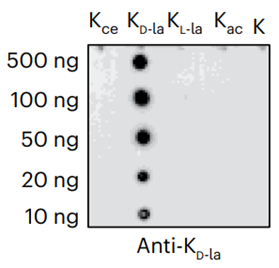
D-lactyllysine (KD-la) refers to the modification of lysine residues in proteins by the addition of D-lactate.dues in proteins. This modification was first reported by Zhang et al. in 2024, with the findings published in Nature Chemical Biology. KD-la forms through a non-enzymatic reaction between proteins and S-D-lactoylglutathione (LGSH), which is a byproduct of the glyoxalase pathway. This pathway involves two key enzymes: glyoxalase 1 (GLO1) and glyoxalase 2 (GLO2). GLO1 conjugates methylglyoxal (MGO), a glycolysis byproduct, with glutathione to form LGSH, which is hydrolyzed by GLO2 to produce D-lactate while regenerating cellular glutathione. In humans, L-lactate is a common metabolic product that plays a key role in energy metabolism, especially during muscle activity. In contrast, D-lactate is not a typical product of human metabolism but is mainly produced by microorganisms such as certain bacteria and yeasts during fermentation. The potential harm of D-lactate mainly lies in its accumulation under specific conditions, such as intestinal microbiota dysbiosis, short bowel syndrome, or certain disease states. Excessive production of D-lactate can lead to D-lactic acidosis, characterized by symptoms of acidosis (low blood bicarbonate levels, decreased blood pH, and hyperuricemia), and in severe cases, neurological symptoms like seizures, ataxia, and altered consciousness.
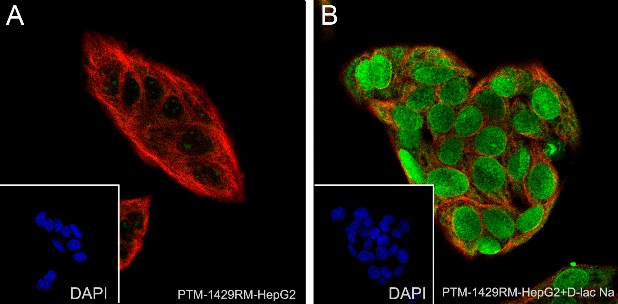
Cellular location
/