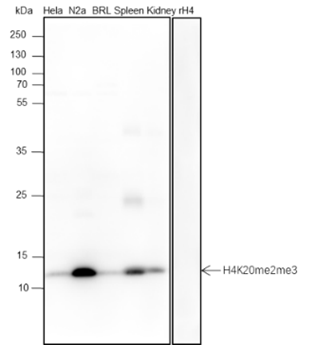
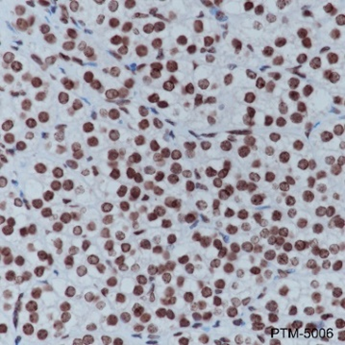
Background
The ε-amino lysine acetylation of proteins is an important reversible modification controlling protein activity. The amino-terminal tails of core histones undergo lysine methylation in multiple sites, termed as “histone code” or “epigenetic code”. Lysine methylation in core histones is a major determinant for the formation of active and inactive regions of the genome and therefore plays vital roles in multiple cellular events. In most species, lysine methylation occurs primarily on histones H3 (Lys4, 9, 27, 36, 79) and H4 (Lys20) and has been implicated in both transcriptional activation and silencing. Methylation in histones modulated by specific histone methyltransferases (HMTs) and histone demethylases (HDMs) is impaired in the pathologies of cancer and other diseases and therefore, enzymes regulating histone lysine methylation have become promising targets for anti-cancer drugs.
Cellular location
Nucleus