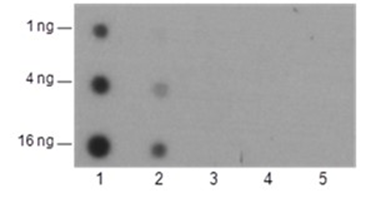
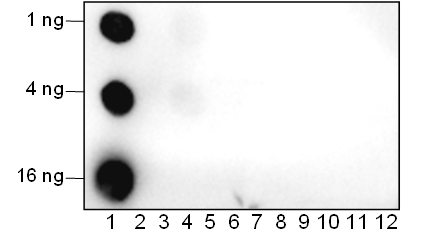
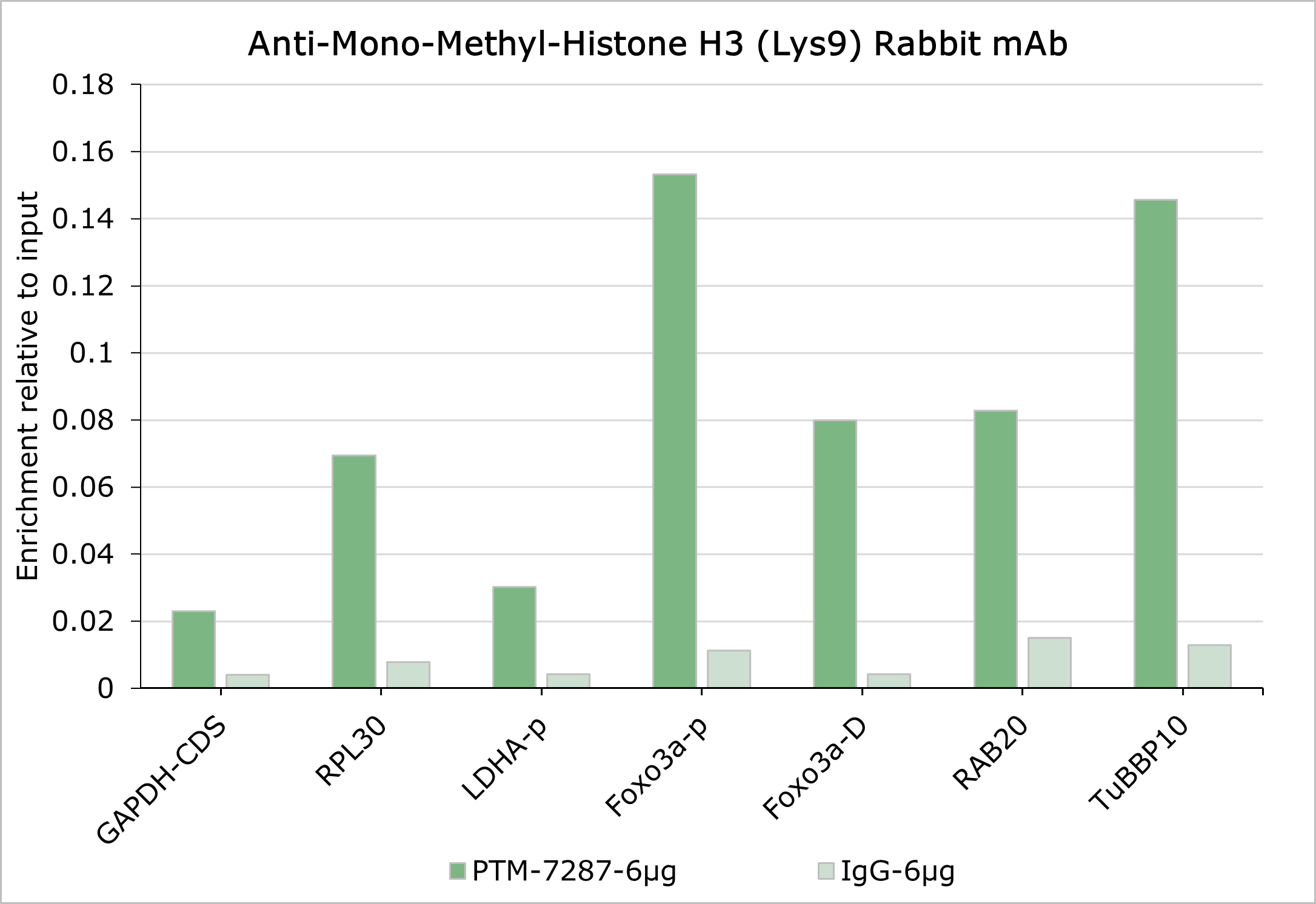
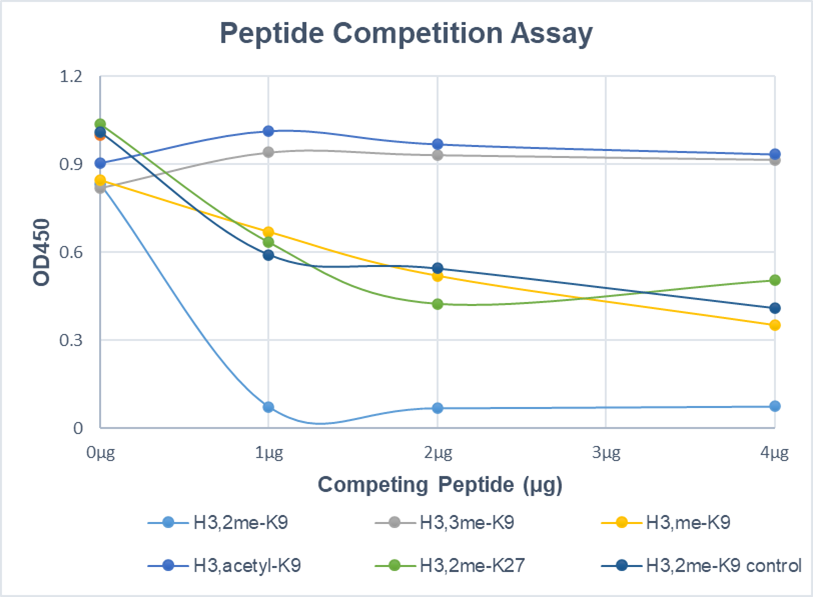
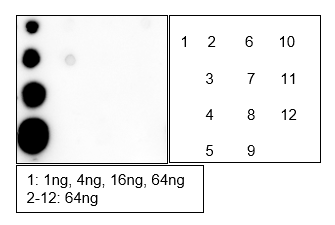
Background
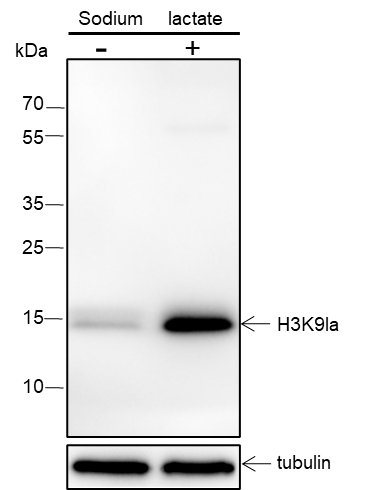
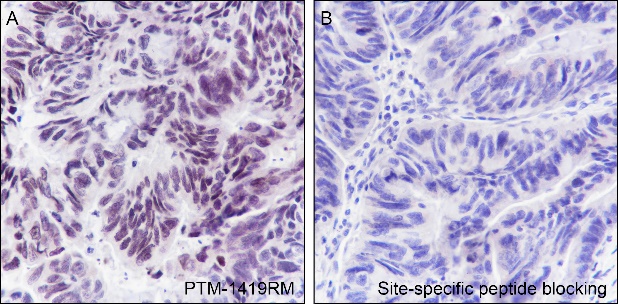
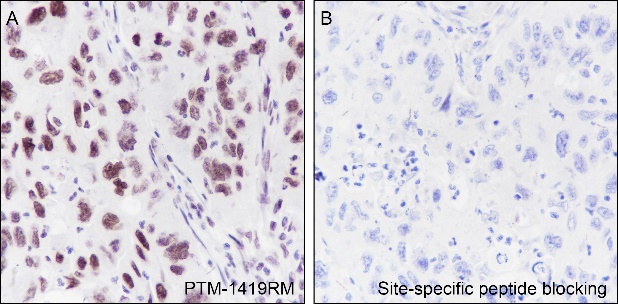
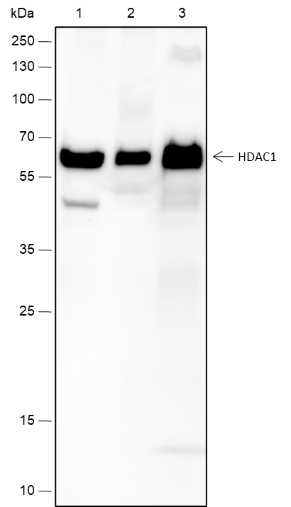
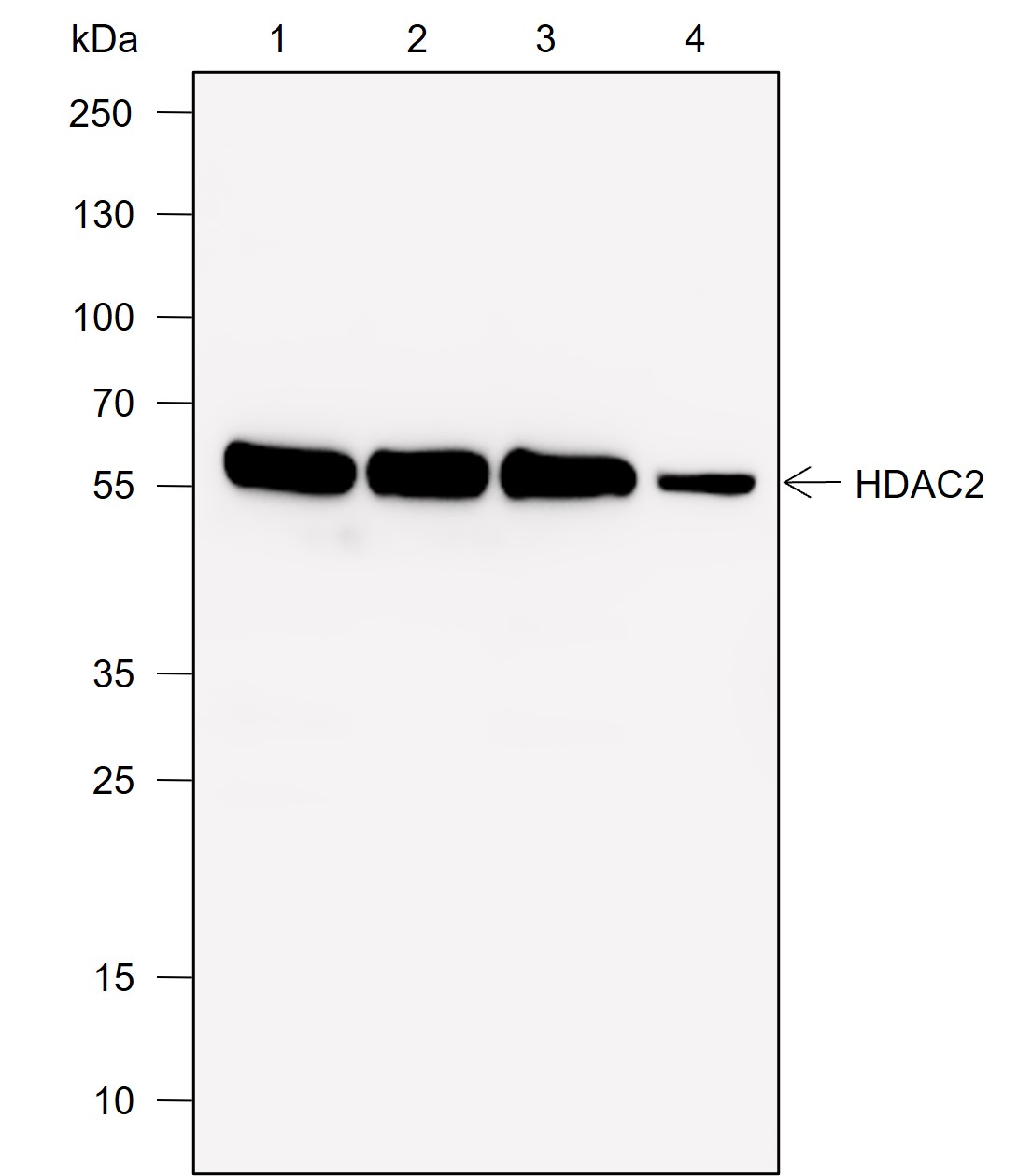
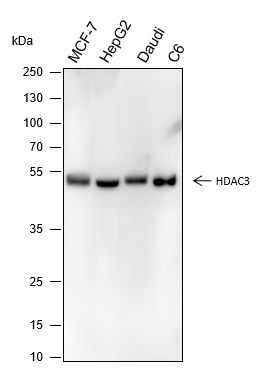
Histones, fundamental proteins involved in chromatin structure and gene regulation, are subject to a wide array of enzyme-catalyzed modifications, including acetylation, methylation, phosphorylation, ubiquitination, and numerous others. Histone L-lactylation, a recently discovered post-translational modification induced by lactate has emerged as a significant addition to this repertoire. The extent and dynamics of this modification are highly reliant on lactate levels within the cellular microenvironment and can be modulated through the introduction of extracellular lactate in cultured cells or the stimulation of intracellular glycolysis. The introduction of lysine L-lactylation is mediated by the acetyltransferase p300, while the removal of lactylation marks from histones has been attributed to Class I histone deacetylases (HDAC 1-3). H3K9 lactylation (H3K9la) has been implicated in tumorigenesis, particularly in hepatocellular carcinoma (HCC), where it plays a role in disease progression. Aberrant levels of H3K9la have been observed in HCC and positively correlate with the expression of markers of cancer malignancy, such as CD133, BCL2, Ki67, and LDHA.
Cellular location
Nucleus