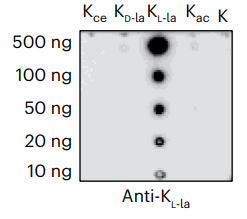
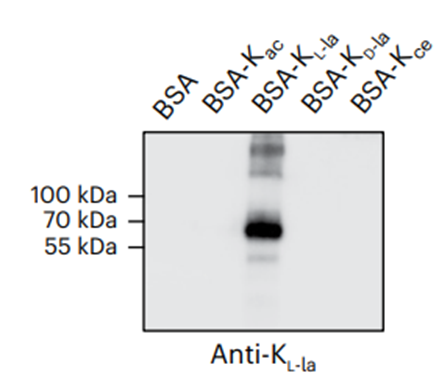
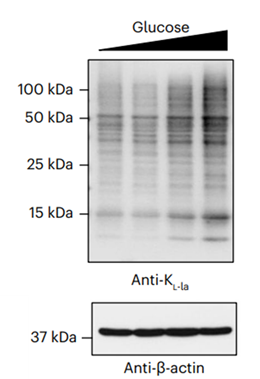
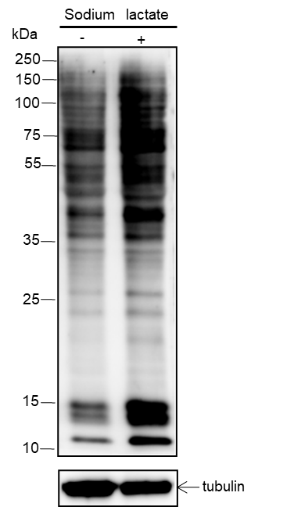
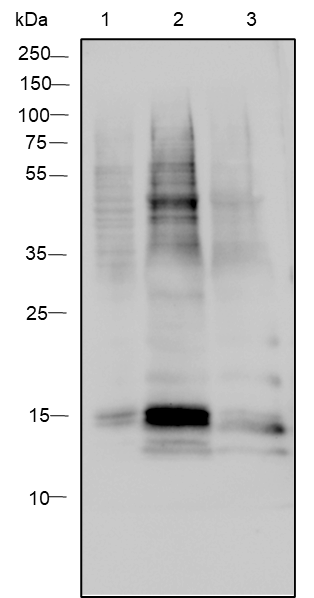
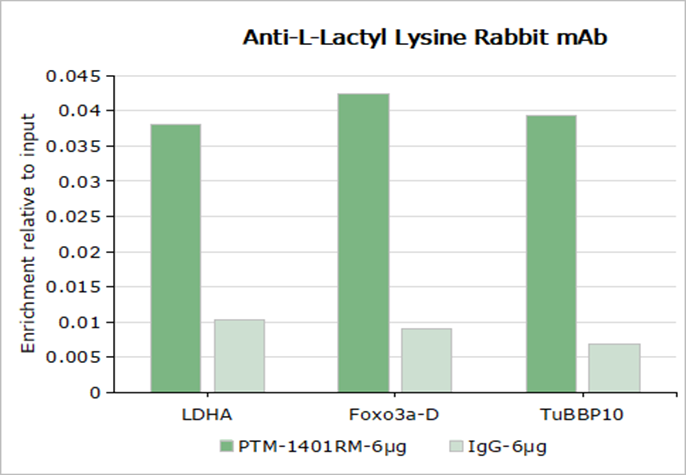
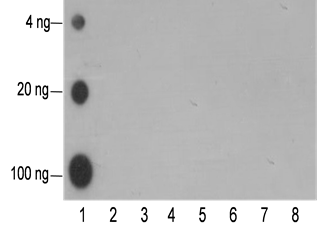
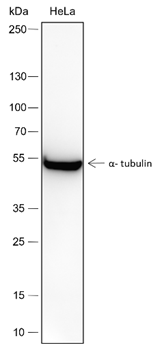
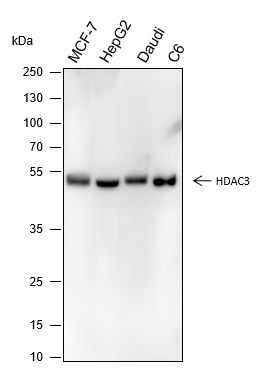
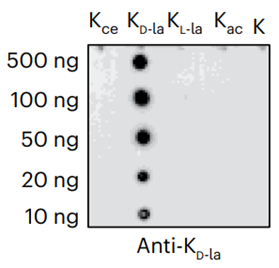
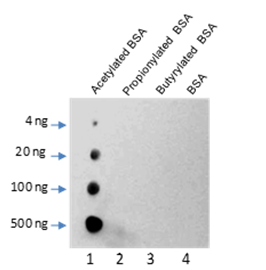
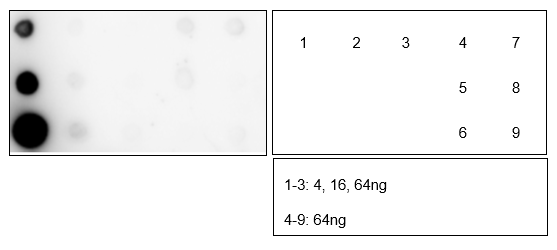
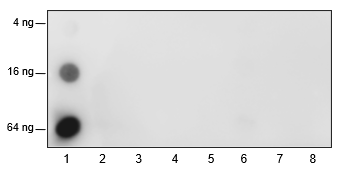
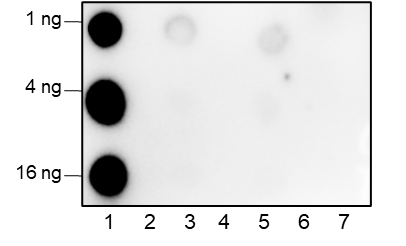
-
Lactylation of Histone H3k18 and Egr1 Promotes Endothelial Glycocalyx Degradation in Sepsis-Induced Acute Lung Injury
-
Year
2024
-
Journal
Advanced Science
-
Authors
Zongqing Lu, et al.
-
Applications
Unspecified
-
Reactivity
-
DNAJC12 downregulation induces neuroblastoma progression via increased histone H4K5 lactylation
-
Year
2024
-
Journal
Journal of Molecular Cell Biology
-
Authors
Yang Yaqi, et al.
-
Applications
Unspecified
-
Reactivity
-
NSUN2 lactylation drives cancer cell resistance to ferroptosis through enhancing GCLC-dependent glutathione synthesis
-
Year
2024
-
Journal
Redox Biology
-
Authors
Kaifeng Niu, et al.
-
Applications
Unspecified
-
Reactivity
-
Nuclear GTPSCS functions as a lactyl-CoA synthetase to promote histone lactylation and gliomagenesis
-
Year
2024
-
Journal
Cell Metabolism
-
Authors
Ruilong Liu, et al.
-
Applications
Unspecified
-
Reactivity
-
β-Hydroxybutyrate suppresses M1 macrophage polarization through β-hydroxybutyrylation of the STAT1 protein
-
Year
2024
-
Journal
Cell Death & Disease
-
Authors
Bai Ya-Ping, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylation-related gene signature accurately predicts prognosis and immunotherapy response in gastric cancer
-
Year
2024
-
Journal
Frontiers in Oncology
-
Authors
Xuezeng Sun, et al.
-
Applications
Unspecified
-
Reactivity
-
Dual activation of GCGR/GLP1R signaling ameliorates intestinal fibrosis via metabolic regulation of histone H3K9 lactylation in epithelial cells
-
Year
2024
-
Journal
Acta Pharmaceutica Sinica B
-
Authors
Han Liu, et al.
-
Applications
Unspecified
-
Reactivity
-
Subcellular Proteomic Mapping of Lysine Lactylation
-
Year
2024
-
Journal
JOURNAL OF THE AMERICAN SOCIETY FOR MASS SPECTROMETRY
-
Authors
Qiuyu Bao, et al.
-
Applications
Unspecified
-
Reactivity
-
Post-translational toxin modification by lactate controls Staphylococcus aureus virulence
-
Year
2024
-
Journal
Nature Communications
-
Authors
Wang Yanan, et al.
-
Applications
Unspecified
-
Reactivity
-
Tufm lactylation regulates neuronal apoptosis by modulating mitophagy in traumatic brain injury
-
Year
2024
-
Journal
CELL DEATH AND DIFFERENTIATION
-
Authors
Weng Weiji, et al.
-
Applications
Unspecified
-
Reactivity
-
RHOF promotes Snail1 lactylation by enhancing PKM2-mediated glycolysis to induce pancreatic cancer cell endothelial–mesenchymal transition
-
Year
2024
-
Journal
Cancer & Metabolism
-
Authors
Zhao Rui, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylation-Driven IGF2BP3-Mediated Serine Metabolism Reprogramming and RNA m6A—Modification Promotes Lenvatinib Resistance in HCC
-
Year
2024
-
Journal
Advanced Science
-
Authors
Yuanxiang Lu, et al.
-
Applications
Unspecified
-
Reactivity
-
GLP-1R activation attenuates the progression of pulmonary fibrosis via disrupting NLRP3 inflammasome/PFKFB3-driven glycolysis interaction and histone lactylation
-
Year
2024
-
Journal
Journal of Translational Medicine
-
Authors
Liu Chenyang, et al.
-
Applications
Unspecified
-
Reactivity
-
Multi-omics reveals lactylation-driven regulatory mechanisms promoting tumor progression in oral squamous cell carcinoma
-
Year
2024
-
Journal
GENOME BIOLOGY
-
Authors
Jing Fengyang, et al.
-
Applications
Unspecified
-
Reactivity
-
Systematic Analysis of Lysine Lactylation in Nucleus Pulposus Cells
-
Year
2024
-
Journal
iScience
-
Authors
Lei Sheng, et al.
-
Applications
Unspecified
-
Reactivity
-
AARS1 and AARS2 sense l-lactate to regulate cGAS as global lysine lactyltransferases
-
Year
2024
-
Journal
NATURE
-
Authors
Li Heyu, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylation drives hCG-triggered luteinization in hypoxic granulosa cells
-
Year
2024
-
Journal
INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES
-
Authors
Gang Wu, et al.
-
Applications
Unspecified
-
Reactivity
-
The effect of Tau K677 lactylation on ferritinophagy and ferroptosis in Alzheimer's disease
-
Year
2024
-
Journal
FREE RADICAL BIOLOGY AND MEDICINE
-
Authors
Xiaoqiong An, et al.
-
Applications
Unspecified
-
Reactivity
-
Sirtuin 1/sirtuin 3 are robust lysine delactylases and sirtuin 1-mediated delactylation regulates glycolysis
-
Year
2024
-
Journal
iScience
-
Authors
Runhua Du, et al.
-
Applications
Unspecified
-
Reactivity
-
Single-cell RNA sequencing integrated with bulk RNA sequencing analysis reveals the protective effects of lactate-mediated lactylation of microglia-related proteins on spinal cord injury
-
Year
2024
-
Journal
CNS Neuroscience & Therapeutics
-
Authors
Bin Zhang, et al.
-
Applications
Unspecified
-
Reactivity
-
H3K9 lactylation in malignant cells facilitates CD8+ T cell dysfunction and poor immunotherapy response
-
Year
2024
-
Journal
Cell Reports
-
Authors
Ruijie Wang, et al.
-
Applications
Unspecified
-
Reactivity
-
Hypoxia conduces the glioma progression by inducing M2 macrophage polarization via elevating TNFSF9 level in a histone-lactylation-dependent manner
-
Year
2024
-
Journal
AMERICAN JOURNAL OF PHYSIOLOGY-CELL PHYSIOLOGY
-
Authors
Min Li, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylation of histone by BRD4 regulates astrocyte polarization after experimental subarachnoid hemorrhage
-
Year
2024
-
Journal
Journal of Neuroinflammation
-
Authors
Zhang Fan, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylome Analysis Reveals Potential Target Modified Proteins in the Retina of Form-deprivation Myopia
-
Year
2024
-
Journal
iScience
-
Authors
Jiaojiao Feng, et al.
-
Applications
Unspecified
-
Reactivity
-
Global profiling of protein lactylation in microglia in experimental high-altitude cerebral edema
-
Year
2024
-
Journal
Cell Communication and Signaling
-
Authors
Jiang Xiufang, et al.
-
Applications
Unspecified
-
Reactivity
-
Lysine l-lactylation is the dominant lactylation isomer induced by glycolysis
-
Year
2024
-
Journal
Nature Chemical Biology
-
Authors
Zhang Di, et al.
-
Applications
Unspecified
-
Reactivity
-
Lysine lactylation-based insight to understanding the characterization of cervical cancer
-
Year
2024
-
Journal
BIOCHIMICA ET BIOPHYSICA ACTA-MOLECULAR BASIS OF DISEASE
-
Authors
Chaoran He, et al.
-
Applications
Unspecified
-
Reactivity
-
Integrative Analysis of Lactylome and Proteome of Hypertrophic Scar To Identify Pathways or Proteins Associated with Disease Development
-
Year
2024
-
Journal
JOURNAL OF PROTEOME RESEARCH
-
Authors
Enyuan Zhang, et al.
-
Applications
Unspecified
-
Reactivity
-
Histone Lactylation Dynamics: Unlocking the Triad of Metabolism, Epigenetics, and Immune Regulation in Metastatic Cascade of Pancreatic Cancer
-
Year
2024
-
Journal
CANCER LETTERS
-
Authors
Xing Wang, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylated Apolipoprotein C-II Induces Immunotherapy Resistance by Promoting Extracellular Lipolysis
-
Year
2024
-
Journal
Advanced Science
-
Authors
Jian Chen, et al.
-
Applications
Unspecified
-
Reactivity
-
Targeting the transmembrane cytokine co-receptor neuropilin-1 in distal tubules improves renal injury and fibrosis
-
Year
2024
-
Journal
Nature Communications
-
Authors
Li Yinzheng, et al.
-
Applications
Unspecified
-
Reactivity
-
Methyl-CpG-binding 2 K271 lactylation-mediated M2 macrophage polarization inhibits atherosclerosis
-
Year
2024
-
Journal
Theranostics
-
Authors
Liangqi Chen, et al.
-
Applications
Unspecified
-
Reactivity
-
Astrocyte-derived lactate aggravates brain injury of ischemic stroke in mice by promoting the formation of protein lactylation
-
Year
2024
-
Journal
Theranostics
-
Authors
Xiao-Yi Xiong, et al.
-
Applications
Unspecified
-
Reactivity
-
p53 lysine-lactylated modification contributes to lipopolysaccharide-induced proinflammatory activation in BV2 cell under hypoxic conditions
-
Year
2024
-
Journal
NEUROCHEMISTRY INTERNATIONAL
-
Authors
Xuechao Fei, et al.
-
Applications
Unspecified
-
Reactivity
-
Comprehensive Proteome and Acetylome Analysis of Needle Senescence in Larix gmelinii
-
Year
2024
-
Journal
INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES
-
Authors
Xuting Zhang, et al.
-
Applications
Unspecified
-
Reactivity
-
Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation
-
Year
2024
-
Journal
Cell Metabolism
-
Authors
Jian Zhou, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactate drives epithelial-mesenchymal transition in diabetic kidney disease via the H3K14la/KLF5 pathway
-
Year
2024
-
Journal
Redox Biology
-
Authors
Xuanxuan Zhang, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylation of NAT10 promotes N4‐acetylcytidine modification on tRNASer-CGA-1-1 to boost oncogenic DNA virus KSHV reactivation
-
Year
2024
-
Journal
CELL DEATH AND DIFFERENTIATION
-
Authors
Yan Qin, et al.
-
Applications
Unspecified
-
Reactivity
-
Protein Lactylation Profiles Provide Insights into Molecular Mechanisms Underlying Metabolism in Yak
-
Year
2024
-
Journal
JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY
-
Authors
Zhijuan Wu, et al.
-
Applications
Unspecified
-
Reactivity
-
Integration of Epigenome and Lactylome Reveals the Regulation of Lipid Production in Nannochloropsis oceanica
-
Year
2024
-
Journal
JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY
-
Authors
Lingyu Ouyang, et al.
-
Applications
Unspecified
-
Reactivity
-
Histone H3K18 and Ezrin Lactylation Promote Renal Dysfunction in Sepsis-Associated Acute Kidney Injury
-
Year
2024
-
Journal
Advanced Science
-
Authors
Jiao Qiao, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylome analyses suggest systematic lysine-lactylated substrates in oral squamous cell carcinoma under normoxia and hypoxia
-
Year
2024
-
Journal
CELLULAR SIGNALLING
-
Authors
Fan Song, et al.
-
Applications
Unspecified
-
Reactivity
-
EPB41L4A-AS1 is required to maintain basal autophagy to modulates Aβ clearance
-
Year
2024
-
Journal
npj Aging
-
Authors
Wang Ziqiang, et al.
-
Applications
Unspecified
-
Reactivity
-
Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma
-
Year
2024
-
Journal
IMMUNITY
-
Authors
Alessandra De Leo, et al.
-
Applications
Unspecified
-
Reactivity
-
Highly expressed lncRNA H19 in endometriosis promotes aerobic glycolysis and Histone lactylation
-
Year
2024
-
Journal
REPRODUCTION
-
Authors
Xiaoyang Wen, et al.
-
Applications
Unspecified
-
Reactivity
-
Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis
-
Year
2024
-
Journal
CELL
-
Authors
Zhi Zong, et al.
-
Applications
Unspecified
-
Reactivity
-
Exercise training decreases lactylation and prevents myocardial ischemia–reperfusion injury by inhibiting YTHDF2
-
Year
2024
-
Journal
BASIC RESEARCH IN CARDIOLOGY
-
Authors
Xu Gui-e, et al.
-
Applications
Unspecified
-
Reactivity
-
The mechanism of PFK-1 in the occurrence and development of bladder cancer by regulating ZEB1 lactylation
-
Year
2024
-
Journal
BMC Urology
-
Authors
Wang Rong, et al.
-
Applications
Unspecified
-
Reactivity
-
Dexamethasone protects against asthma via regulating Hif-1α-glycolysis-lactate axis and protein lactylation
-
Year
2024
-
Journal
INTERNATIONAL IMMUNOPHARMACOLOGY
-
Authors
Ning Chen, et al.
-
Applications
Unspecified
-
Reactivity
-
MUC20 regulated by extrachromosomal circular DNA attenuates proteasome inhibitor resistance of multiple myeloma by modulating cuproptosis
-
Year
2024
-
Journal
JOURNAL OF EXPERIMENTAL & CLINICAL CANCER RESEARCH
-
Authors
Wang Xiaobin, et al.
-
Applications
Unspecified
-
Reactivity
-
Proanthocyanidins Ameliorate LPS-Inhibited Osteogenesis of PDLSCs by Restoring Lysine Lactylation
-
Year
2024
-
Journal
INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES
-
Authors
Yaxin Wu, et al.
-
Applications
Unspecified
-
Reactivity
-
Human papillomavirus-16 E6 activates the pentose phosphate pathway to promote cervical cancer cell proliferation by inhibiting G6PD lactylation
-
Year
2024
-
Journal
Redox Biology
-
Authors
Qingfei Meng, et al.
-
Applications
Unspecified
-
Reactivity
-
Andrographolide regulates H3 histone lactylation by interfering with p300 to alleviate aortic valve calcification
-
Year
2024
-
Journal
BRITISH JOURNAL OF PHARMACOLOGY
-
Authors
Chunli Wang, et al.
-
Applications
Unspecified
-
Reactivity
-
Augmentation of scleral glycolysis promotes myopia through histone lactylation
-
Year
2024
-
Journal
Cell Metabolism
-
Authors
Xiaolei Lin, et al.
-
Applications
Unspecified
-
Reactivity
-
Deciphering the Atlas of Post-Translational Modification in Sugarcane
-
Year
2023
-
Journal
JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY
-
Authors
Qibin Wu, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylome Analyses Suggest Systematic Lysine-Lactylated Substrates in Oral Squamous Cell Carcinoma Under Normoxia and Hypoxia
-
Year
2023
-
Journal
iScience
-
Authors
Song Fan, et al.
-
Applications
Unspecified
-
Reactivity
-
High-concentrate diet elevates histone lactylation mediated by p300/CBP through the upregulation of lactic acid and induces an inflammatory response in mammary gland of dairy cows
-
Year
2023
-
Journal
MICROBIAL PATHOGENESIS
-
Authors
Lairong Wang, et al.
-
Applications
Unspecified
-
Reactivity
-
Integrative analysis of lactylation-related genes and establishment of a novel prognostic signature for hepatocellular carcinoma
-
Year
2023
-
Journal
JOURNAL OF CANCER RESEARCH AND CLINICAL ONCOLOGY
-
Authors
Cai Diankui, et al.
-
Applications
Unspecified
-
Reactivity
-
Global proteomic analysis reveals lysine succinylation contributes to the pathogenesis of aortic aneurysm and dissection
-
Year
2023
-
Journal
Journal of Proteomics
-
Authors
Hongwei Zhang, et al.
-
Applications
Unspecified
-
Reactivity
-
Tandem mass tag-based quantitative proteomic analysis identification of succinylation related proteins in pathogenesis of thoracic aortic aneurysm and aortic dissection
-
Year
2023
-
Journal
PeerJ
-
Authors
Yu Zhang, et al.
-
Applications
Unspecified
-
Reactivity
-
Natural product fargesin interferes with H3 histone lactylation via targeting PKM2 to inhibit non-small cell lung cancer tumorigenesis
-
Year
2023
-
Journal
BIOFACTORS
-
Authors
Zizhang Guo, et al.
-
Applications
Unspecified
-
Reactivity
-
Metabolic regulation of homologous recombination repair by MRE11 lactylation
-
Year
2023
-
Journal
CELL
-
Authors
Yuping Chen, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactate-induced histone lactylation by p300 promotes osteoblast differentiation
-
Year
2023
-
Journal
PLoS One
-
Authors
Erika Minami, et al.
-
Applications
Unspecified
-
Reactivity
-
Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia
-
Year
2023
-
Journal
Immunity & Ageing
-
Authors
Han Hao, et al.
-
Applications
Unspecified
-
Reactivity
-
Histone lactylation promotes cell proliferation, migration and invasion through targeting HMGB1 in endometriosis
-
Year
2023
-
Journal
Journal of Biomedical Research
-
Authors
Jie Chen, et al.
-
Applications
Unspecified
-
Reactivity
-
BZW2 Modulates Lung Adenocarcinoma Progression through Glycolysis-Mediated IDH3G Lactylation Modification
-
Year
2023
-
Journal
JOURNAL OF PROTEOME RESEARCH
-
Authors
Ming Wang, et al.
-
Applications
Unspecified
-
Reactivity
-
Aldolase B-driven lactagenesis and CEACAM6 activation promote cell renewal and chemoresistance in colorectal cancer through the Warburg effect
-
Year
2023
-
Journal
Cell Death & Disease
-
Authors
Chu Yu-De, et al.
-
Applications
Unspecified
-
Reactivity
-
STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia
-
Year
2023
-
Journal
Signal Transduction and Targeted Therapy
-
Authors
Huang Ze-Wei, et al.
-
Applications
Unspecified
-
Reactivity
-
Defective branched-chain amino acid catabolism in dorsal root ganglia contributes to mechanical pain
-
Year
2023
-
Journal
EMBO REPORTS
-
Authors
Huijing Xie, et al.
-
Applications
Unspecified
-
Reactivity
-
H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer's disease through the NFκB signaling pathway
-
Year
2023
-
Journal
Journal of Neuroinflammation
-
Authors
Wei Lin, et al.
-
Applications
Unspecified
-
Reactivity
-
Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites
-
Year
2023
-
Journal
PHYTOMEDICINE
-
Authors
Haiying Xu, et al.
-
Applications
Unspecified
-
Reactivity
-
SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth
-
Year
2023
-
Journal
EMBO REPORTS
-
Authors
Jing Jin, et al.
-
Applications
Unspecified
-
Reactivity
-
A small-molecule cocktail promotes mammalian cardiomyocyte proliferation and heart regeneration
-
Year
2022
-
Journal
Cell Stem Cell
-
Authors
Jianyong Du, et al.
-
Applications
Unspecified
-
Reactivity
-
PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages
-
Year
2022
-
Journal
JOURNAL OF EXPERIMENTAL & CLINICAL CANCER RESEARCH
-
Authors
Wang Lu, et al.
-
Applications
Unspecified
-
Reactivity
-
TFAM loss induces nuclear actin assembly upon mDia2 malonylation to promote liver cancer metastasis
-
Year
2022
-
Journal
EMBO JOURNAL
-
Authors
Qichao Huang, et al.
-
Applications
Unspecified
-
Reactivity
-
Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages
-
Year
2022
-
Journal
International Journal of Biological Sciences
-
Authors
Wang Jizhuang, et al.
-
Applications
Unspecified
-
Reactivity
-
GABA regulates IL-1β production in macrophages
-
Year
2022
-
Journal
Cell Reports
-
Authors
Jian Fu, et al.
-
Applications
Unspecified
-
Reactivity
-
Overexpression of Tfap2a in Mouse Oocytes Impaired Spindle and Chromosome Organization
-
Year
2022
-
Journal
INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES
-
Authors
Juan Lin, et al.
-
Applications
Unspecified
-
Reactivity
-
Global-Scale Profiling of Differential Expressed Lysine-Lactylated Proteins in the Cerebral Endothelium of Cerebral Ischemia–Reperfusion Injury Rats
-
Year
2022
-
Journal
CELLULAR AND MOLECULAR NEUROBIOLOGY
-
Authors
Yao Yuan, et al.
-
Applications
Unspecified
-
Reactivity
-
Integrated insights into the mechanisms underlying sepsis-induced myocardial depression using a quantitative global proteomic analysis
-
Year
2022
-
Journal
Journal of Proteomics
-
Authors
Ni Yang, et al.
-
Applications
Unspecified
-
Reactivity
-
Metabolism-epigenetic interactions on in vitro produced embryos
-
Year
2022
-
Journal
REPRODUCTION FERTILITY AND DEVELOPMENT
-
Authors
Marcella Pecora Milazzotto, et al.
-
Applications
Unspecified
-
Reactivity
-
Protein lactylation induced by neural excitation
-
Year
2021
-
Journal
Cell Reports
-
Authors
Hideo Hagihara, et al.
-
Applications
Unspecified
-
Reactivity
-
Increased lactate in AML blasts upregulates TOX expression, leading to exhaustion of CD8+ cytolytic T cells
-
Year
2021
-
Journal
American Journal of Cancer Research
-
Authors
Ying Chen, et al.
-
Applications
Unspecified
-
Reactivity