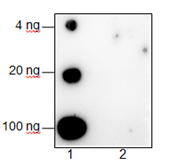
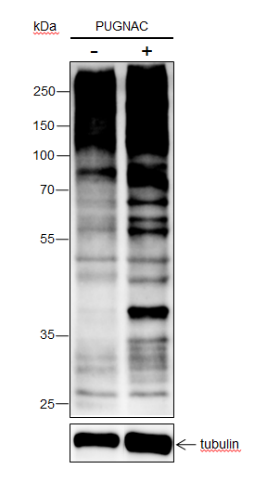
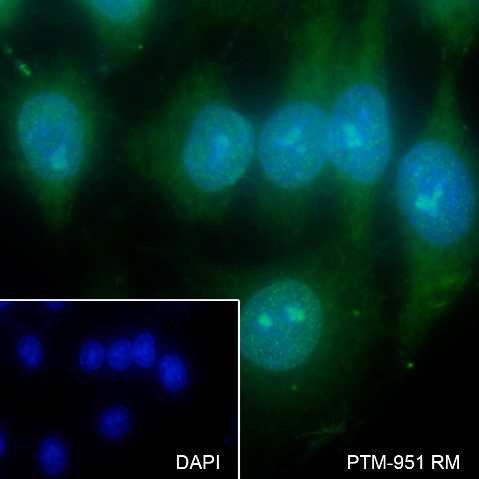
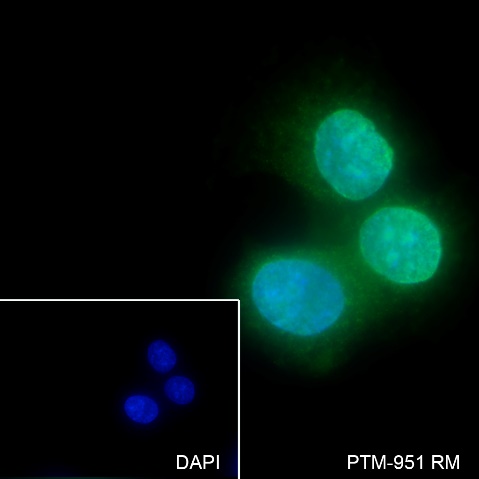
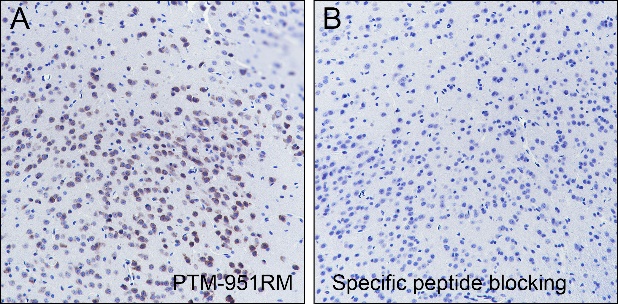
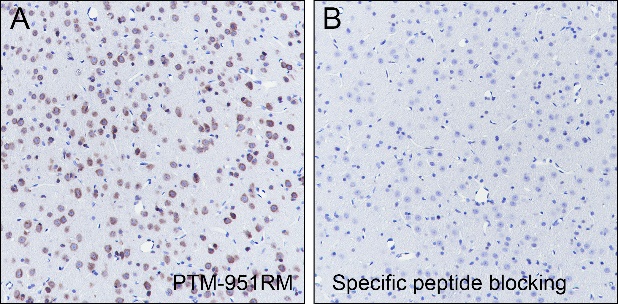
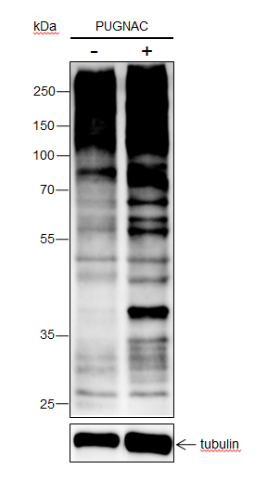
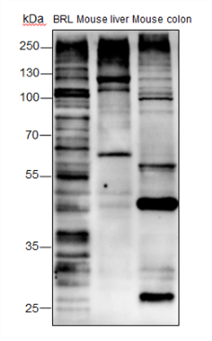
Background
O-Linked β-N-acetylglucosamine modification (O-GlcNAcylation) is a glycosylation in which the monosaccharide β-N-acetylglucosamine (GlcNAc) is attached to serine/threonine residues via an O-linked glycosidic bond. This modification is found mostly within the cytoplasm or nucleoplasm. O-GlcNAcylation regulates several important cellular processes, including signal transduction, protein expression, degradation, trafficking, and embryonic stem cell pluripotency. This post-translational modification is regulated by O-GlcNAc transferases (OGT), which catalyzes the addition of GlcNAc, and β-N-acetylglucosaminidase (O-GlcNAcase), which mediates its removal. O-GlcNAcylation has been identified on histones, including H2A at T101, H2B at S36 and S112, H3 at S10 and T32, and H4 at S47.
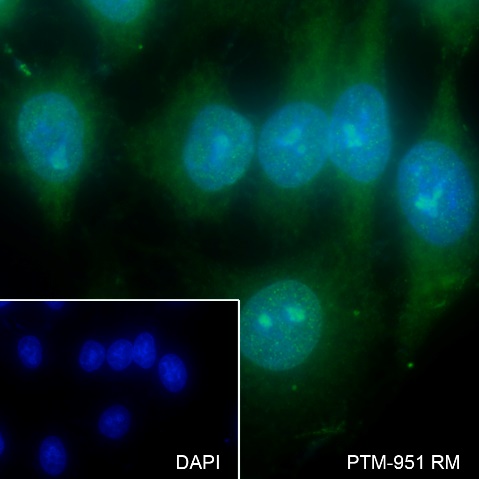
Cellular location
/