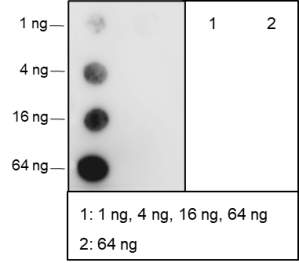
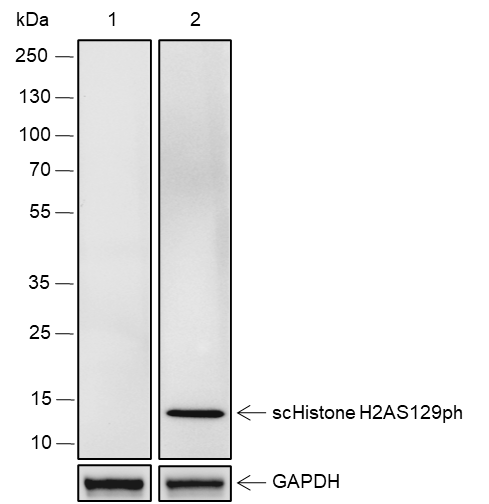
Background
Histone post-translational modifications (PTMs) are key mechanisms of epigenetics that modulate chromatin structures, termed as “histone code”. The PTMs on histone including acetylation, methylation, phosphorylation and novel acylation directly affect the accessibility of chromatin to transcription factors and other epigenetic regulators, altering genome stability, gene transcription, etc. Histone phosphorylation occurs on serine, threonine and tyrosine residues on the aminoterminal of core histones. This histone mark plays roles in DNA repair,transcription and chromatin remodeling. The best-known histone phosphorylation site is histone variant H2A.x Ser139ph, which were reported to be involved in the response to DNA damage. Histone phosphorylation is mainly involved in processes during both mitosis and meiosis. Many kinases and phosphatases regulate histone phosphorylation levels. H2A(X) Ser 129 is essential for survival in the presence of topoisomerase-1-mediated DNA damage.
Cellular location
Nucleus