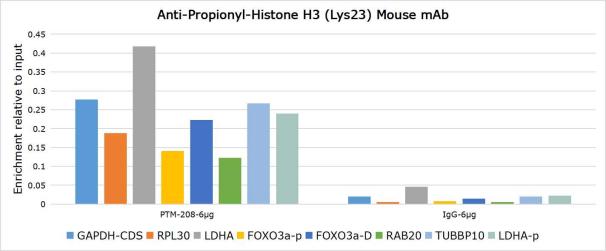
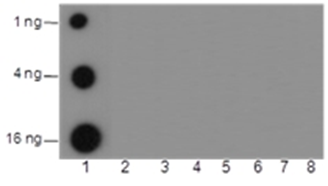
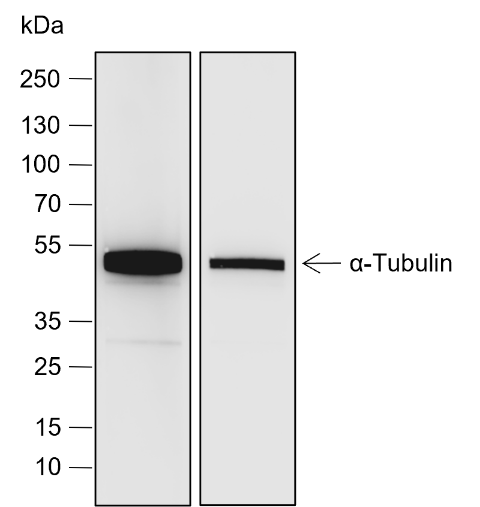
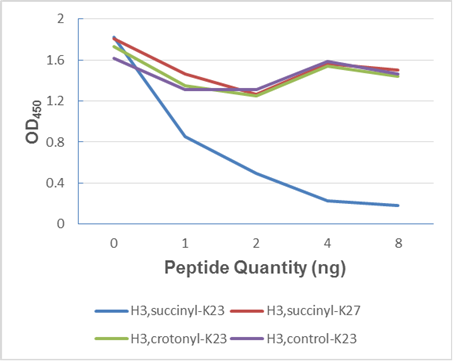
Background
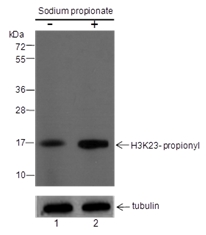
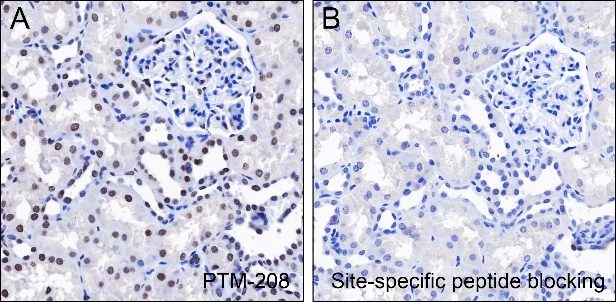
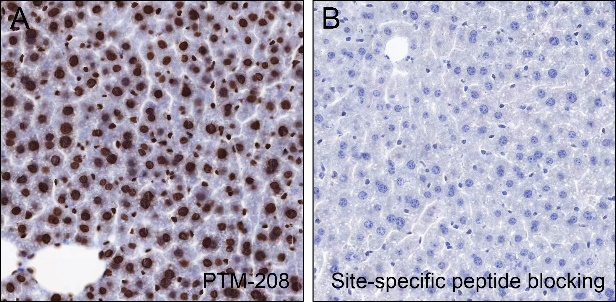
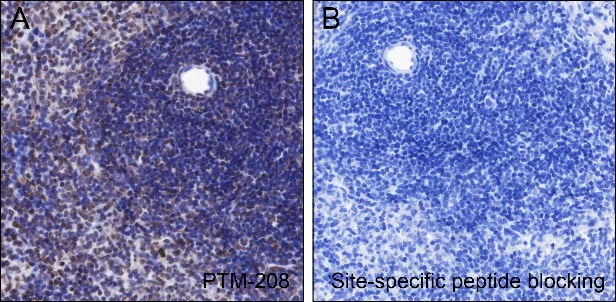
Histones are subject to a variety of enzyme-catalyzed modifications, including acetylation, methylation, phosphorylation, ubiquitylation, and others. Among these modifications, lysine propionylation (Kpr) has emerged as a newly identified reversible modification that plays a crucial role in controlling protein activity. Structurally similar to lysine acetylation, lysine propionylation is prevalent in both prokaryotes and eukaryotes and has been identified in a wide range of proteins, including histones and non-histone substrates like p53. Recent research has unveiled the involvement of stoichiometric complexes comprising lysine acetyltransferase 6A (KAT6A), KAT6B, and bromodomain- and PHD finger-containing protein 1 (BRPF1) in the catalysis of histone H3 Lys23 propionylation (H3K23pr). Importantly, deficiencies in histone propionylation have been associated with developmental disorders and cancer.
Cellular location
Nucleus