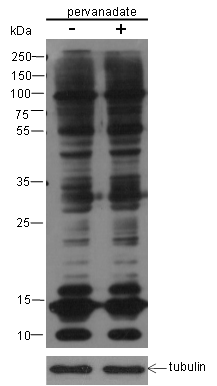
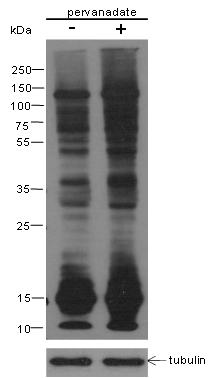
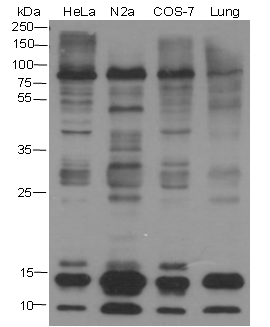
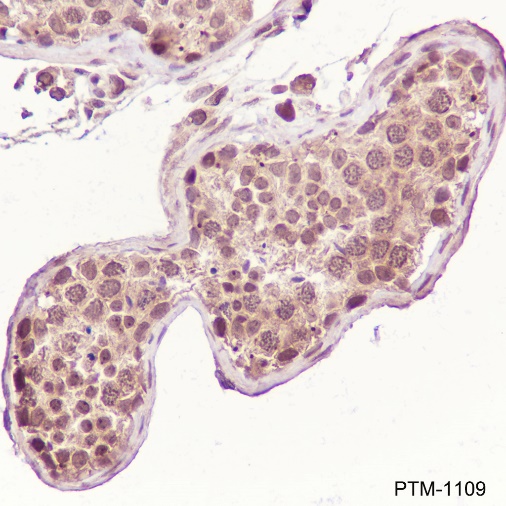
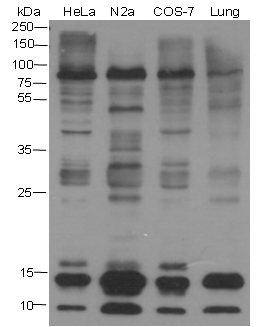
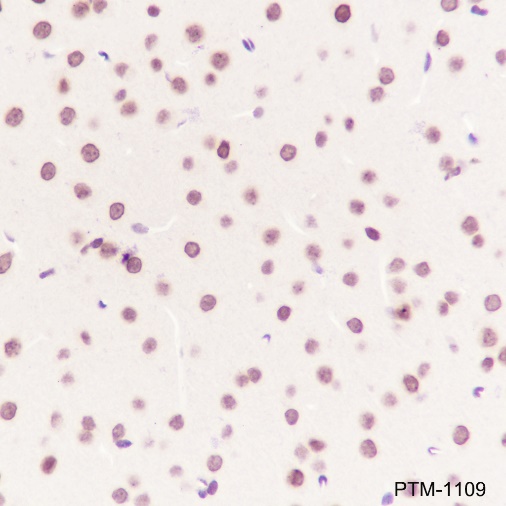
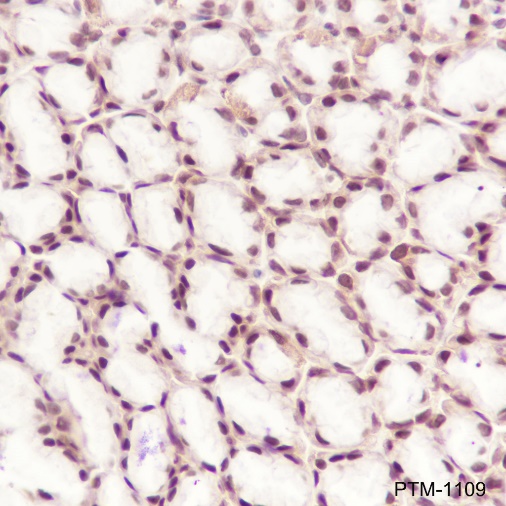
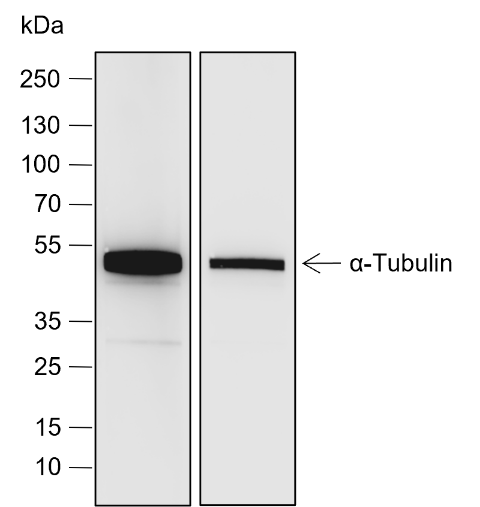
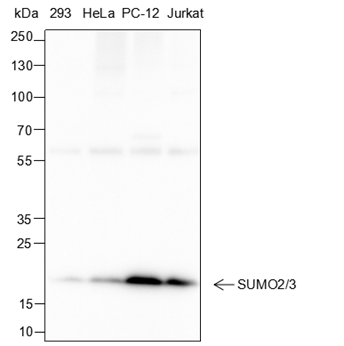
Background
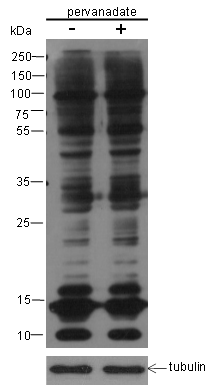
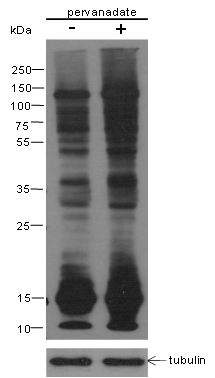
Small ubiquitin-related modifier 1, 2 and 3 (SUMO-1, 2 and 3) are members of the ubiquitin-like protein family. The functions of the small ubiquitin-related modifier protein is similar to ubiquitin in that it is bound to target proteins as part of a post-translational modification system. It can be covalently attached to proteins as a monomer or a lysine-linked polymer. Covalent attachment via an isopeptide bond to its substrates requires prior activation by the E1 complex SAE1-SAE2 and linkage to the E2 enzyme UBE2I and can be promoted by E3 ligases such as PIAS1-4, RANBP2 or CBX4. Ubiquitin predominantly regulates degradation of its target. In contrast, SUMO-1 is conjugated to RanGAP, PML, p53 and IκB-α to regulate nuclear trafficking, formation of subnuclear structures, transcriptional activity, and protein stability. SUMO-2/3 forms poly-(SUMO) chains, which is conjugated to topoisomerase II and APP, and regulates chromosomal segregation and cellular responses to environmental stress.
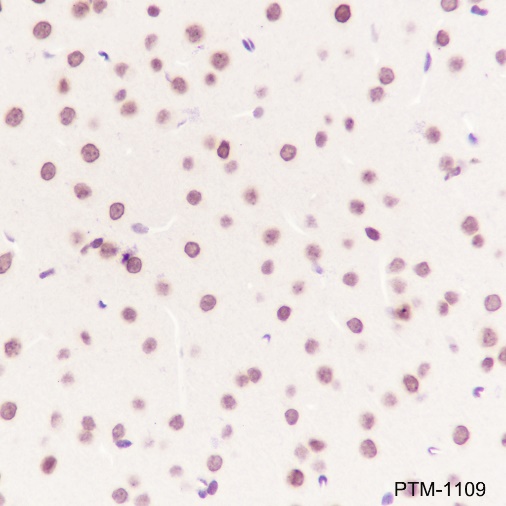
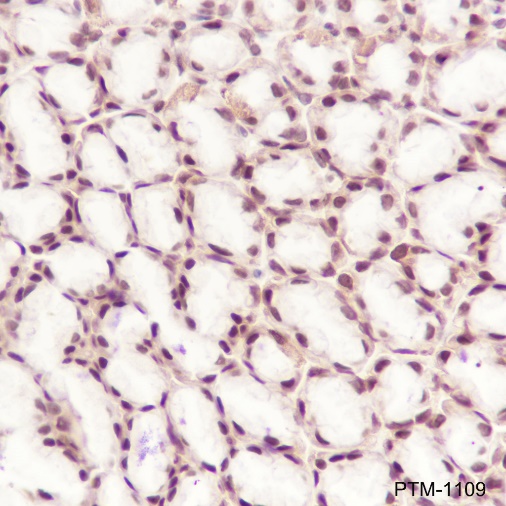
Cellular location
Nucleus, Cytoplasm