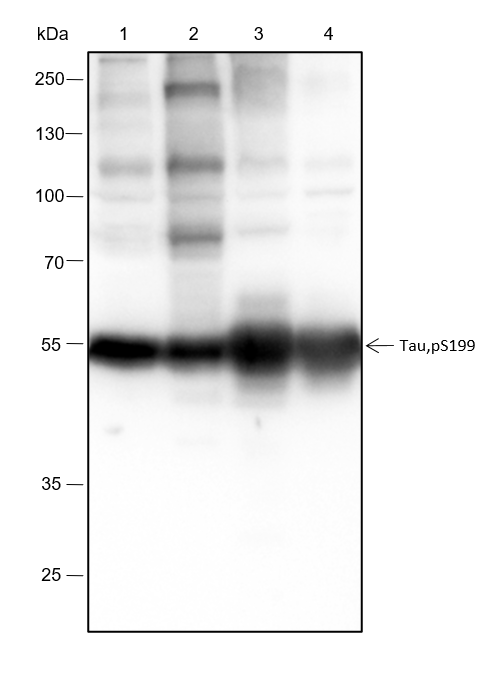
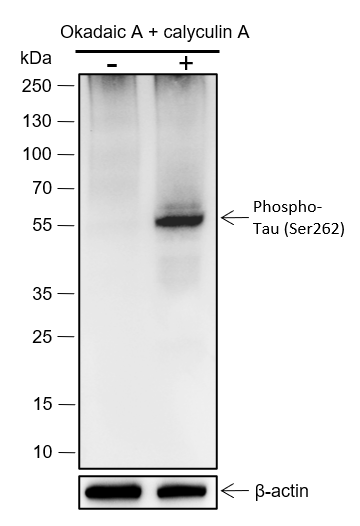
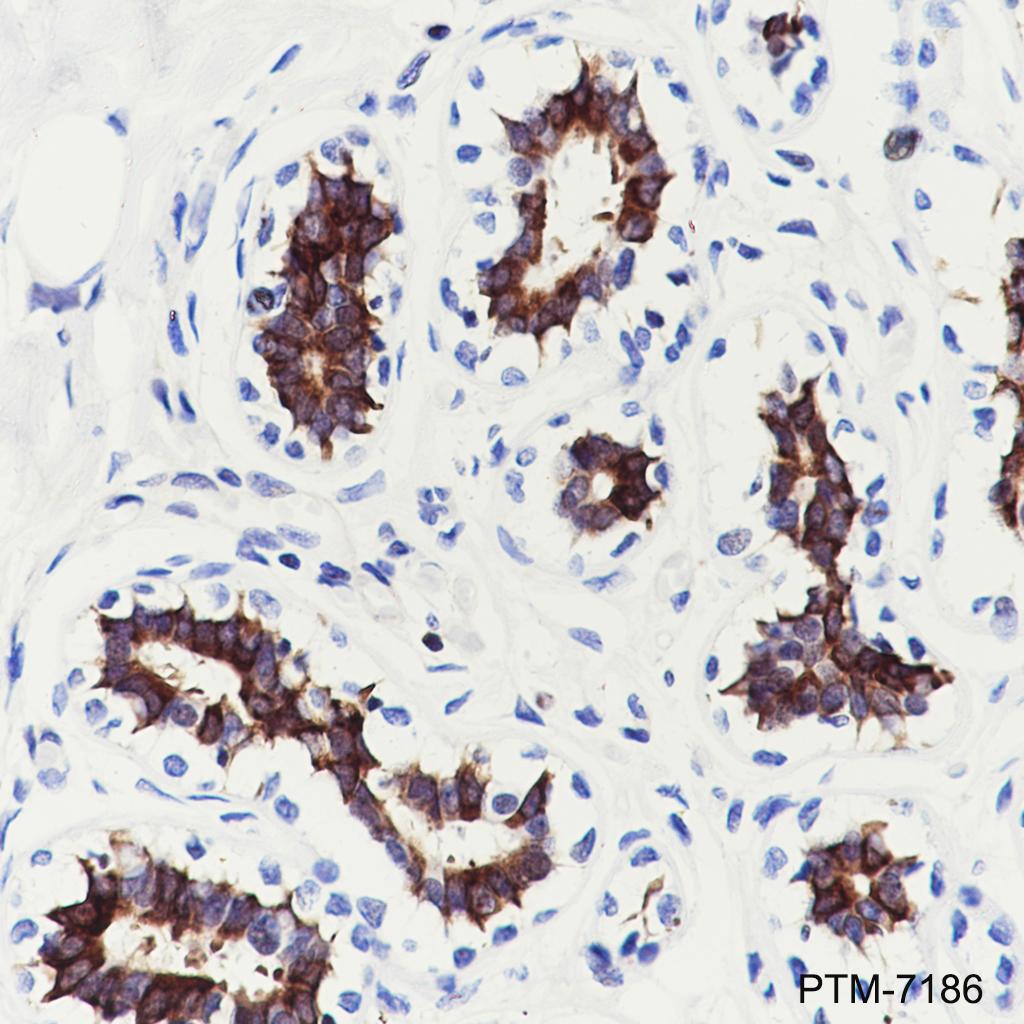
Background
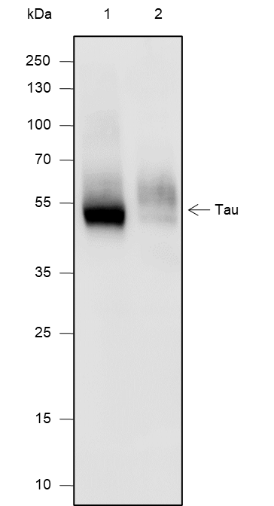
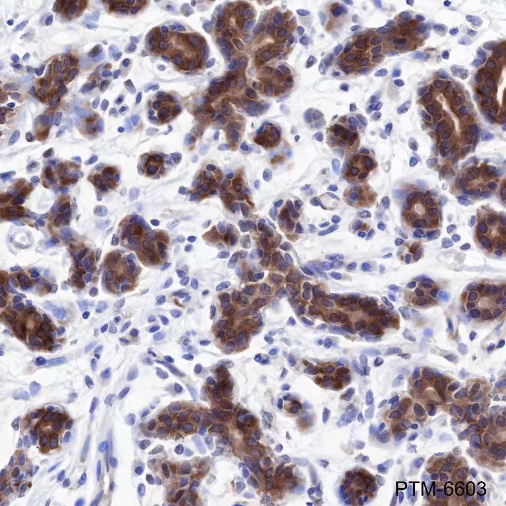
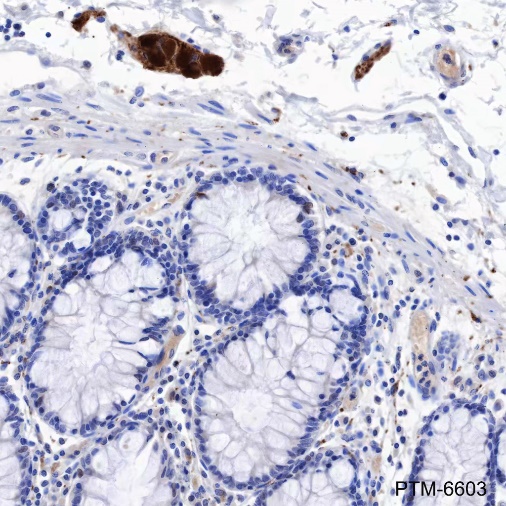
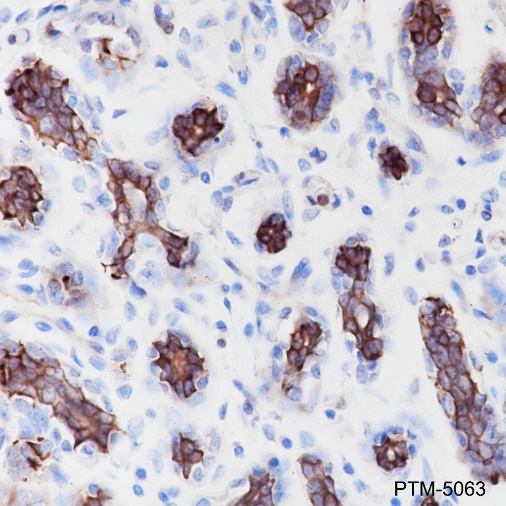
Tau is a heterogeneous microtubule-associated protein that promotes and stabilizes microtubule assembly, especially in axons. Six isoforms with different amino-terminal inserts and different numbers of tandem repeats near the carboxy terminus have been identified, and tau is hyperphosphorylated at approximately 25 sites by Erk, GSK-3, and CDK5. Phosphorylation decreases the ability of tau to bind to microtubules. Neurofibrillary tangles are a major hallmark of Alzheimer's disease (AD); these tangles are bundles of paired helical filaments (PHFs) composed of hyperphosphorylated tau. In particular, phosphorylation at Ser396 by GSK-3 or CDK5 destabilizes microtubules. Furthermore, research studies have shown that inclusions of tau are found in a number of other neurodegenerative diseases, collectively known as tauopathies.
Cellular location
Plasma membrane, Cytoplasm, secreted, axon