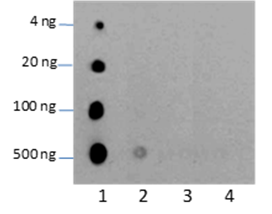
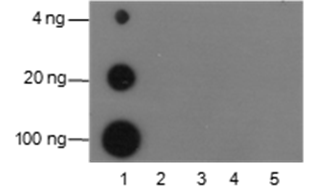
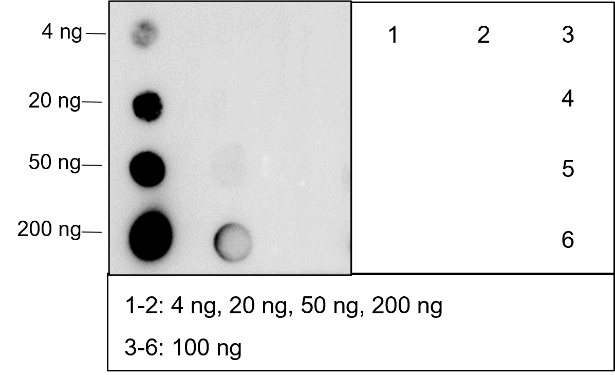
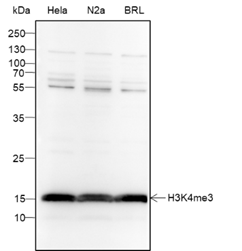
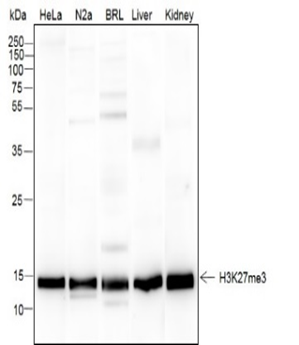
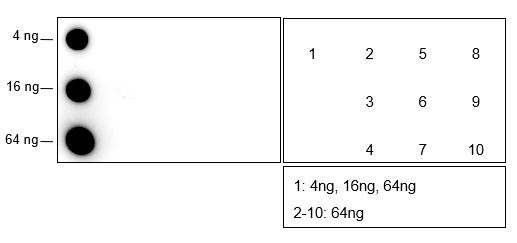
Background
Histone post-translational modifications (PTMs), known as the “histone code”, are key mechanisms of epigenetics that modulate chromatin structures. The PTMs on histone including acetylation, methylation, phosphorylation and novel acylations directly affect the accessibility of chromatin to transcription factors and other epigenetic regulators, altering genome stability and gene transcription. Histone methylation primarily occurs at lysine and arginine residues on the amino terminus of core histones. The methylation of histones can either enhance or repress gene transcription, depending on the specific amino acids (Lys or Arg) being methylated and the number of attached methyl groups (mono-, di-, or trimethylation for Lys; mono-di-symmetric/asymmetric methylation for Arg). Lysine methylation is commonly observed at histone H3 Lys residues 4, 9, 27, 36, 79, and histone H4 Lys20, while Arginine methylation predominantly occurs at histone H3 Arg residues 2, 8, 17, 26, and histone H4 Arg3. The regulation of histone methylation is primarily governed by histone methyltransferases (HMTs) and histone demethylases (HDMs).
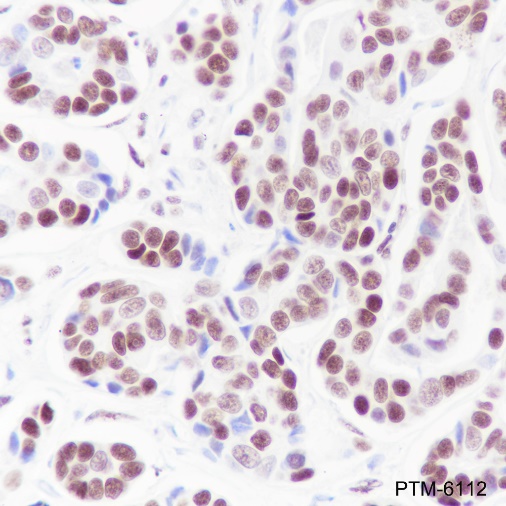
Cellular location
/