Clone Number: /
Host: Rabbit Clonality: Polyclonal
Applications: WB IHC-P ICC/IF
Reactivity: Human, Mouse, Rat, Monkey
Synonyms: /
Shipping: Ambient temperature
Order online or send purchase order to info@ptmbio.com

| Isotype | IgG |
| Conjugate | Unconjugated |
| Synonyms | / |
| UniProt ID | |
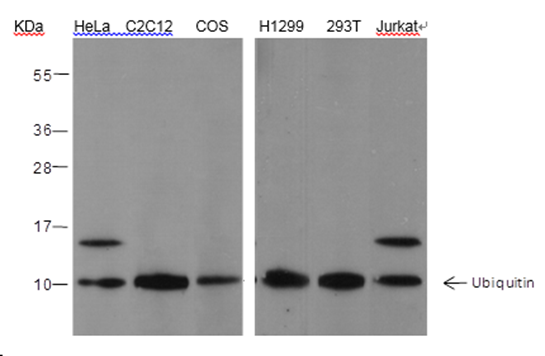
| Immunogen | Synthetic peptide corresponding to the N-terminus of human ubiquitin |
| MW (kDa) | Multiple |
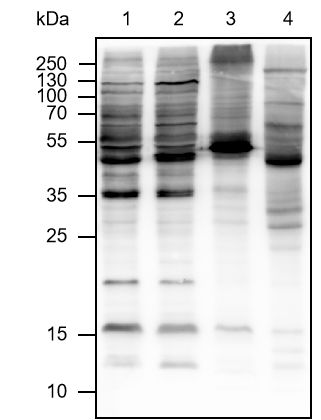
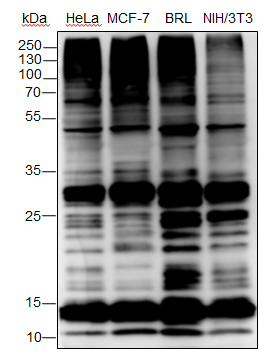
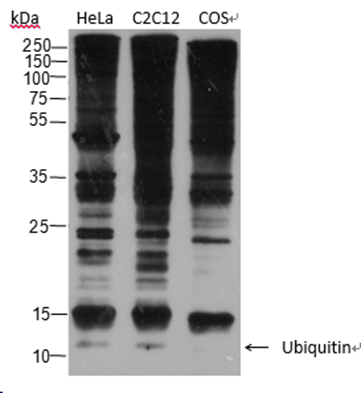
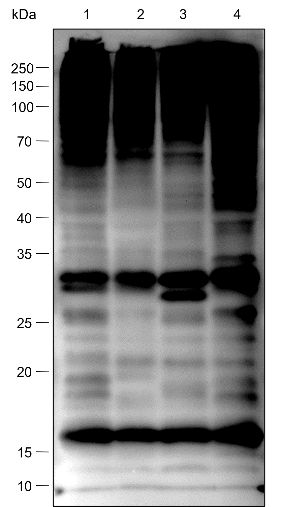
| Specificity | Anti-Ubiquitin Rabbit pAb (N-terminal) detects endogenous ubiquitin, polyubiquitin, and ubiquitinated proteins. |
| Applications | Dilution | Recommended Species |
|---|---|---|
| WB | 1:500 - 1:2000 | Human, Mouse, Rat, Monkey |
| IHC-P | 1:50 - 1:200 | Human |
| ICC/IF | 1:50 - 1:200 | Human |
| Purity | Protein A and immunogen affinity purified |
| Constituents | PBS, Glycerol, BSA |
| Storage | Store at -20°C. Avoid freeze/thaw cycles. |
| Stability | Stable for 12 months from date of receipt/reconstitution. |
Background
Ubiquitin (Ub) is a highly conserved 76-amino acid protein that plays a critical role in regulating cellular processes. By covalently attaching to target proteins through a three-step process involving Ub-activating (E1), Ub-conjugating (E2), and Ub-ligating (E3) enzymes, ubiquitination marks the target proteins for proteasomal degradation, modulates membrane protein trafficking, alters protein-protein interactions, and controls the activity of many signal transduction pathways. Ubiquitination occurs through the formation of an isopeptide bond between its C-terminal Gly76 and a lysine residue in the target protein. This process can occur either as a monomer (monoubiquitin) or as a polymer (polyubiquitin chains), where the C-terminus of a chain extending ubiquitin becomes linked to the N-terminus (M1) or one of seven Lys residues (Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, and Lys63) within a substrate-bound ubiquitin molecule, resulting in polyubiquitin chains with different functions. Lys6-linked may be involved in DNA repair; Lys11-linked is involved in ERAD (endoplasmic reticulum-associated degradation) and in cell-cycle regulation; Lys29-linked is involved in lysosomal degradation; Lys33-linked is involved in kinase modification; Lys48-linked is involved in protein degradation via the proteasome; Lys63-linked is involved in endocytosis, and DNA-damage responses.
Cellular location
Nucleus, Cytoplasm
| WB | |
|---|---|

|
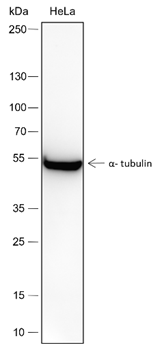
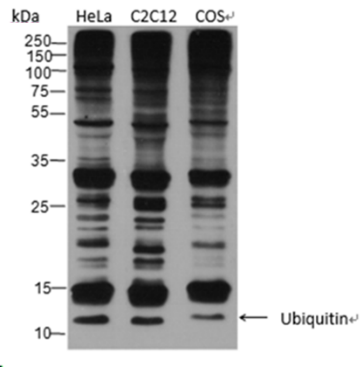
Lysates: HeLa, C2C12, and COS cells |
| IHC-P | |
|---|---|
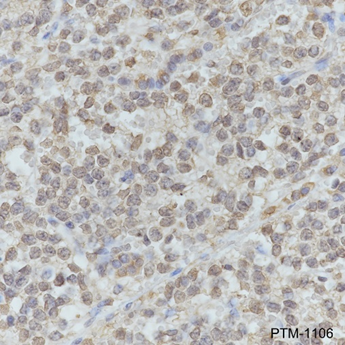
|
Tissue: Human neuroblastoma |
| ICC/IF | |
|---|---|
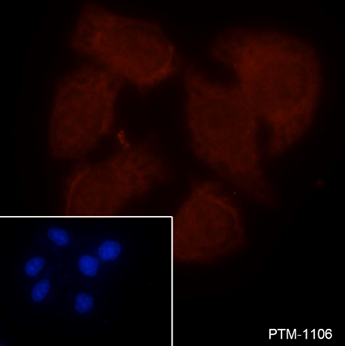
|
Samples: HeLa cells |
Research Use
For research use only, not for use in diagnostic procedures.