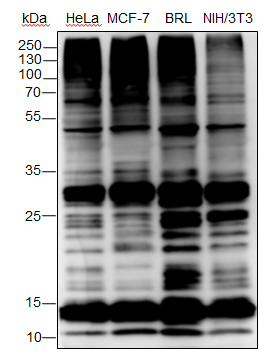
Background
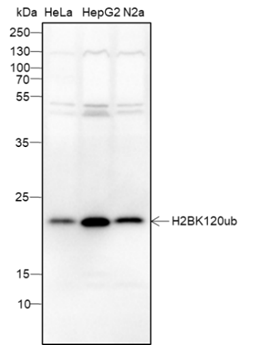
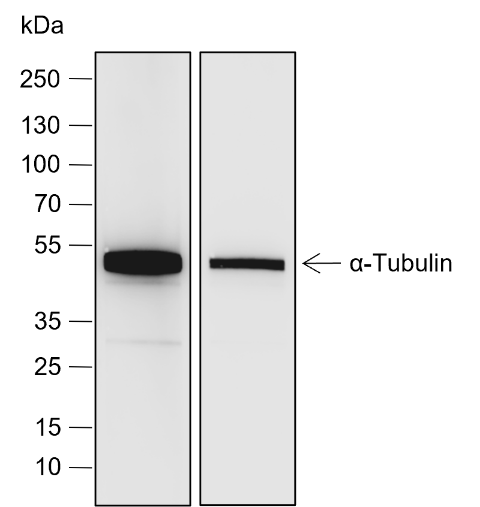
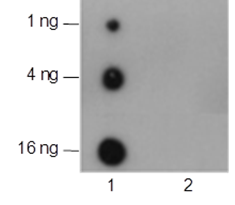
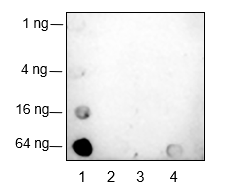
Ubiquitin (Ub) is a highly conserved 76-amino acid protein that plays a critical role in regulating a wide range of cellular processes. Through a three-step process involving Ub-activating (E1), Ub-conjugating (E2), and Ub-ligating (E3) enzymes, ubiquitin covalently attaches to target proteins and marks them for proteasomal degradation, alters protein-protein interactions, modulates membrane protein trafficking, and controls the activity of many signal transduction pathways. Mono-ubiquitination of Histone H2B at Lys120 (H2BK120ub) serves signaling purposes and is reversible by ubiquitin-specific proteases (USPs). The enzymes responsible for H2BK120ub are Rad6 E2 proteins (HR6A and HR6B in human) and Bre1 E3 ligase (RNF20 and RNF40 in human). This modification triggers a “trans-histone” modification cascade, resulting in trimethylation of Lys4 and Lys79 in histone H3, which promotes gene activity.
Cellular location
Nucleus