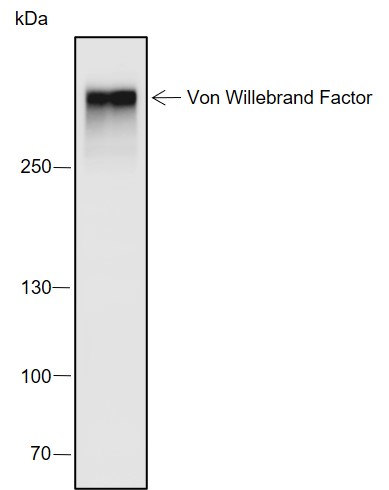
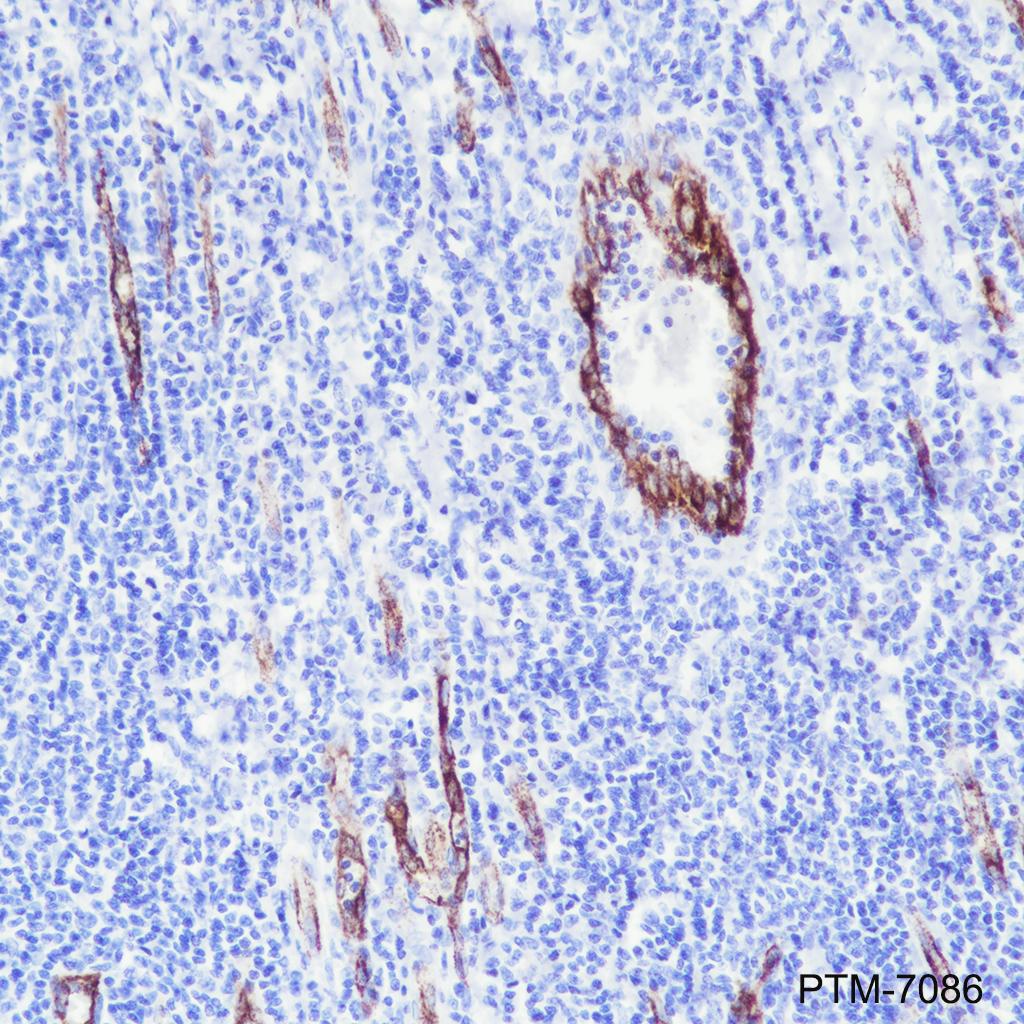
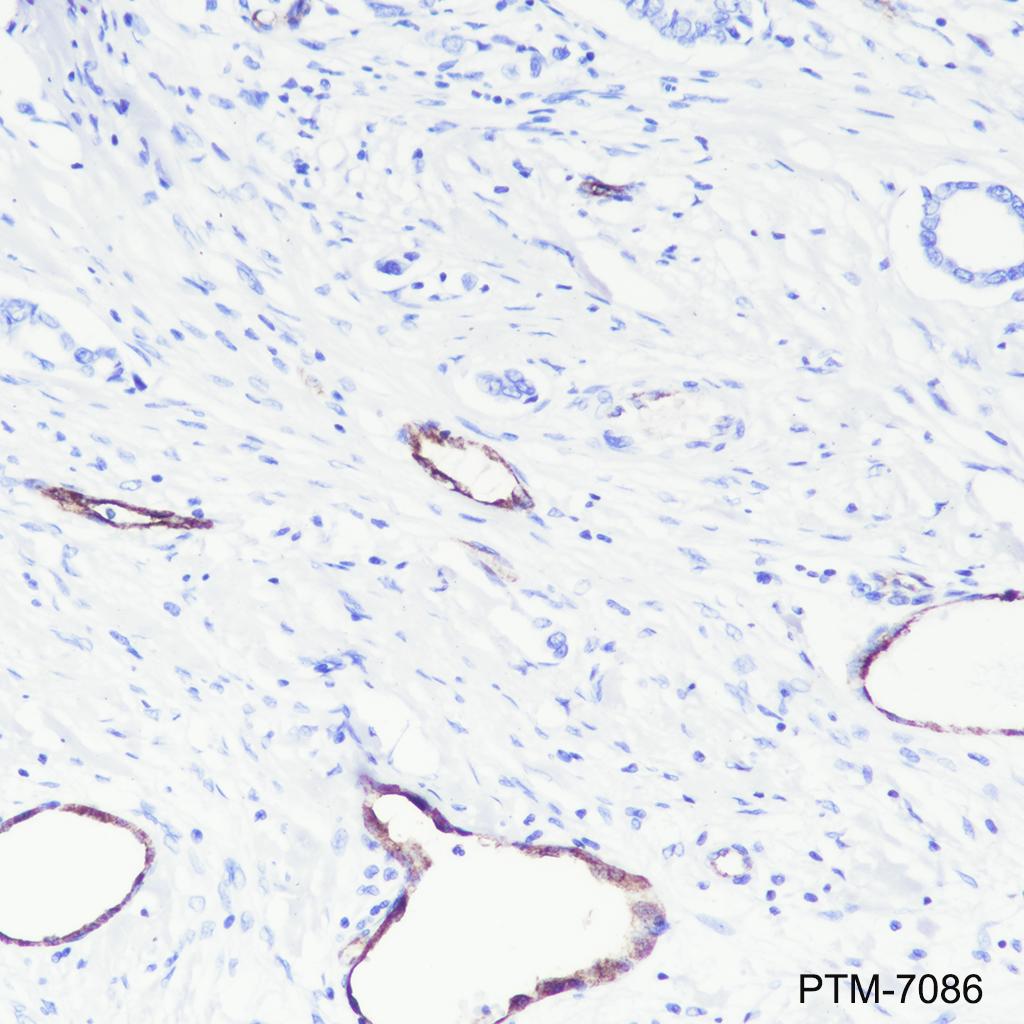
Background
VWF (Von Willebrand factor) is a multimeric plasma glycoprotein that promotes adhesion of platelets to sites of vascular injury. Mature circulating VWF is made up of disulfide-bonded multimers that are in a complex with factor VIII. VWF is stored in secretory Weibel-Palade bodies in endothelial cells. It is synthesized as a large precursor protein and undergoes extensive posttranslational modifications including dimerization in the endoplasmic reticulum followed by cleavage of the pro-peptide and multimerization in the Golgi apparatus. VWF is important in hemostasis, and genetic defects in the structure and modification of VWF can cause von Willebrand disease (VWD), the most common congenital bleeding disorder in humans. Alternatively, increased levels of VWF have been shown to be involved in acute coronary thrombosis and are a clinical risk marker for atherosclerosis.
Cellular location
Secreted