Interested in our services
Contact our experts to provide further information



Additionally, protein cysteine modifications play crucial roles in many biological processes, including redox signaling, enzyme catalysis, protein folding, and protein-protein interactions. Moreover, aberrant cysteine modifications have been linked to various diseases, such as cancer, neurodegeneration, and cardiovascular diseases. Therefore, studying cysteine modifications and their regulation mechanisms is of great significance in understanding the physiological and pathological processes of biological systems.
Cysteine redox modification, similar to other significant post-translational modifications (e.g., phosphorylation, acetylation, ubiquitination), is capable of regulating protein activity, interaction, and localization in a reversible manner, thus achieving precise regulation of numerous biological processes. Under normal physiological conditions, cysteine redox maintains a dynamic balance; however, imbalances have been linked to several major diseases, including cancer, diabetes, and neurodegenerative disorders. To further investigate the physiological and pathological importance of cysteine redox modification in a systematic manner, extensive measurements of its formation and changes during key physiological activities or the progression of major diseases are necessary.
Protein modification technology offers a "wide-angle mirror" for observing cysteine redox conversions in a "Data-Driven" mode. However, the primary challenge in developing this technology is the highly unstable chemical and dynamic biological nature of cysteine redox states. Chemical strategies are typically employed to "capture" and "enrich" the modifications, followed by mass spectrometry for qualitative and quantitative analysis.
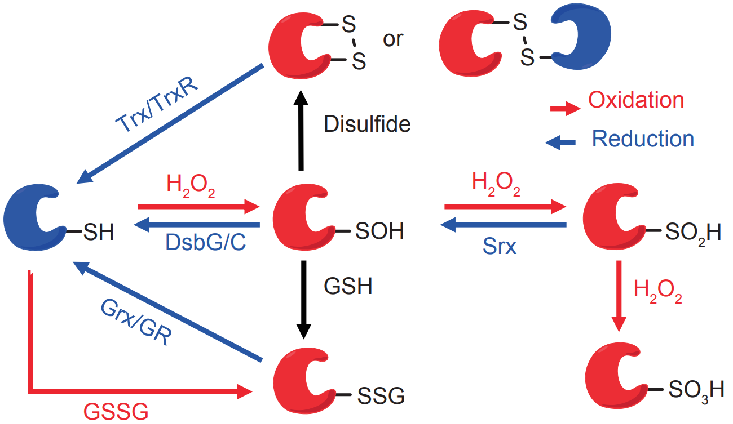
The PTM BIO cysteine redox modification omics technology allows for site identification and quantitative analysis of peroxynitrite and glutathione modifications. Its strategy involves selective reduction, wherein all reduced sulfhydryl groups on the proteome are first blocked, followed by selective reduction of reversible oxidative modifications to sulfhydryl groups using a chemical or enzymatic system. Finally, functional sulfhydryl reactive reagents (such as iodo TMT or isobaric iodo-acetyl tandem mass spectrometry labeling) or solid-phase carriers (such as the resin-assisted capture method) are used for enrichment, and the samples are loaded into liquid chromatography tandem mass spectrometry for analysis. By retrieving corresponding databases, matching and biogenic analysis, information on identification sites and quantitative results can be obtained.
1. Nat Commun: Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages
In this study, researchers located and quantitatively analyzed s-glutathionated protein based on iodotmt redox modification omics of PTM BIO, and clarified the molecular mechanism of glutathionated FABP5 at cys127 site in macrophages to alleviate acute lung injury by exerting its anti-inflammatory function. The results provide a new theoretical basis for the regulation mechanism of fatty acid metabolism in acute lung injury.
2.Nat Commun: Global profiling of distinct cysteine redox forms reveals wide-ranging redox regulation in C. elegans
This study used cysteine redox modification omics to globally characterize the redox proteome of Caenorhabditis elegans, revealing that cysteine redox modification can regulate a variety of biological processes and pathways in nematodes. It was found that proteins upstream and downstream of p38 MAPK pathway can mediate cysteine dependent antioxidant response and natural immune regulation. This also provides rich and valuable data resources for the field of redox biology.
3. Plant Cell Environ: Proteome-wide identification of S-sulfenylated cysteines in Brassica napus
In this study, the redox modification omics of PTM BIO was used. It was found that there were 912 redox modified proteins in Brassica napus under salt stress, and the changes of sulfinylation modification level were observed, and the increase of oxidation level in vivo was observed during salt stress. The results increase our understanding of protein redox modification landscape under stress, and provide ideas and basis for the understanding of redox regulation network.
4. Nat Cell Biol: Cholesterol and fatty acids regulate cysteine ubiquitylation of ACAT2 through competitive oxidation
Researchers combined protein mass spectrometry and various biochemical experiments to reveal that cysteine at position 277 of cholesterol acyltransferase ACAT2 can be oxidized by ROS produced by lipid accumulation, inhibiting ubiquitination at this site, and delaying protein degradation. Therefore, stable ACAT2 protein can acylate and modify excessive polar lipid molecules to reduce its cytotoxicity. This study revealed a special ubiquitination modification: cysteine ubiquitination modification, and revealed its important biological significance.
Interested in our services
Contact our experts to provide further information