Interested in our services
Contact our experts to provide further information



SUMOylation is a newly discovered post-translational modification of proteins. It involves the covalent attachment of small ubiquitin-like modifier (SUMO), a small protein with a molecular weight of approximately 12 kDa, to target proteins via an isopeptide bond between the C-terminal di-glycine of SUMO and the ε-amino group of a lysine residue on the target protein. There are five members in the SUMO family. SUMOylation is a common modification that plays a critical role in numerous important physiological and biochemical processes in cells, including regulation of protein-protein and protein-DNA interactions. It has been linked to the pathogenesis of various diseases, including cardiovascular disease, cancer, neurodegenerative diseases, and immune disorders.
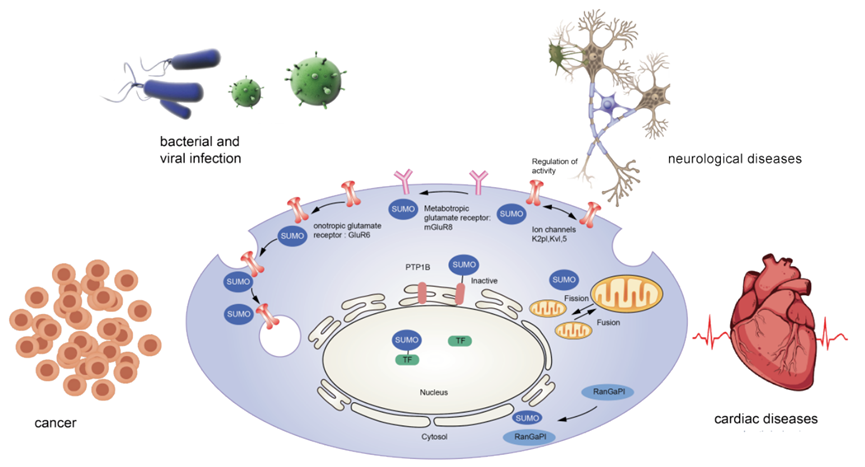
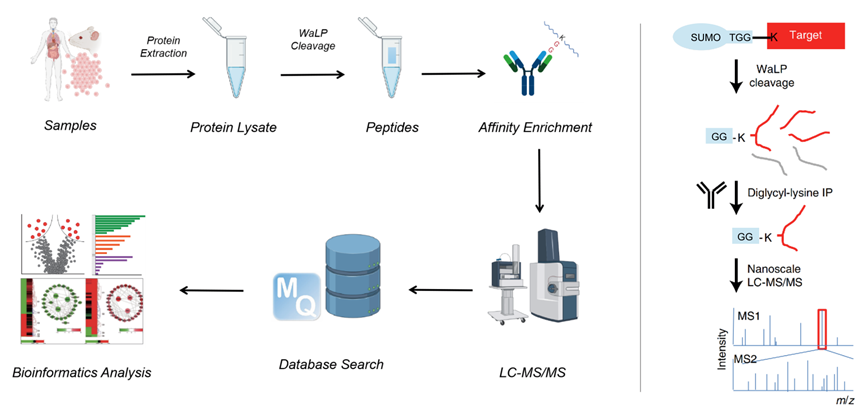
Fig. (left) 4D-sumo modification omics technology flow chart. (right) waLp enzymolysis and high specific K- ε- GG antibody enrichment diagram. The protein was extracted after the cell sample was lysed and enzymolysis was carried out with specific waLp protease. WaLp is a serine endopeptidase, which will specifically cleave the carboxyl terminal side of amino acids such as alanine, serine, threonine and valine to produce double glycine residues. Then high specificity K was used- ε- GG antibody was enriched with SUMO peptide.
PTM BIO has developed 4D-SUMOylation proteomic analysis products by combining the advanced 4D proteomics technology platform. These products enable high-depth and high-accuracy qualitative and quantitative analysis of SUMOylation in various biological samples, such as tissues and cells. The aim is to assist researchers in decrypting the unknown regulatory mechanism of protein SUMOylation and exploring the research areas where the understanding is still shallow.
1. Nature sub journal methodology application, more authoritative and professional!
WaLp enzyme digestion +k-ε-GG antibody enrichment and the site coverage is higher.
2. System information analysis, data, and information deep mining!
Automatic analysis + personalized customization, and deeply excavate the new mechanism of SUMO regulation.
3. A new generation of 4D technology, modification, and identification of higher depth!
Based on timsTOF Pro/timsTOF Pro 2, the identification depth of modification group is greatly improved.
1. SUMOylation activates the antitumor immunity of PDAC:
Gut: Targeting pancreatic cancer byTAK-981: a SUMOylation inhibitor that activates the immune system and blockscancer cell cycle progression in a preclinical model
The potential role of SUMOylation modification in pancreatic cancer was evaluated. It was found that most cancer samples showed high expression level of SUMOylation modification. Based on this, researchers chose a small molecule inhibitor to study the correlation between SUMOylation modification and pancreatic cancer cell proliferation. The results showed that the inhibitor could selectively reduce the level of SUMOylation modification, thereby blocking cell proliferation, leading to mitotic failure and chromosome segregation defects in pancreatic cancer cells.
2. A new mechanism of SUMOylation in response to cell stress:
Sci Adv: Epac1 activation by cAMP regulates cellular SUMOylation and promotes the formation of biomolecular condensates.
Epac1 activation promotes cell SUMOylation modification, camp activation epac1 triggers phase separation and the formation of nuclear aggregates containing general components of epac1 and SUMOylation mechanisms to promote cell SUMOylation. In addition, gene knockout of Epac1 eliminated sumo induced by oxidized low-density lipoprotein in macrophages, thereby inhibiting the formation of foam cells.
3. SUMOylation and stem cell development:
MCP: Identification of SUMO Targets Associated with the Pluripotent State in Human Stem Cells
After treatment of cells with SUMO activator inhibitors, the inhibited SUMOylation will lead to decreased expression and morphological changes of pluripotent markers in cells. Moreover, there are almost no large-scale changes in cell proteome, which means that the loss of SUMOylation in cells may affect the changes of important factors related to nuclear structure and function, and then affect protein abundance, and ultimately affect phenotype.
4. SUMOylation and EGFR signal transduction regulation:
EMBO Reports: Transient deSUMOylation of IRF2BP proteinscontrols early transcription in EGFR signaling
SUMOylation is a molecular switch in epidermal growth factor receptor (EGFR) signal transduction.
5. SUMOylation and neurogenesis:
Cell Death Dis: The Sumo proteome of proliferating and neuronal-differentiating cells reveals Utf1 among key Sumo targets involved in neurogenesis.
This paper analyzes how SUMOylation regulates the function of utf1 (regulating the affinity of chromatin; promoting the binding with decapitate Dcp1α), and reveals its specific functional mechanism as a target protein of SUMOylation related to neurogenesis.
Interested in our services
Contact our experts to provide further information