Interested in our services
Contact our experts to provide further information



Ubiquitylation is a widely observed post-translational modification of proteins, in which one or more molecules of ubiquitin (a 76-amino acid polypeptide) are covalently attached to target proteins within cells, through a series of specialized enzymes. This process helps to classify proteins, select target proteins, and make specific modifications to them.
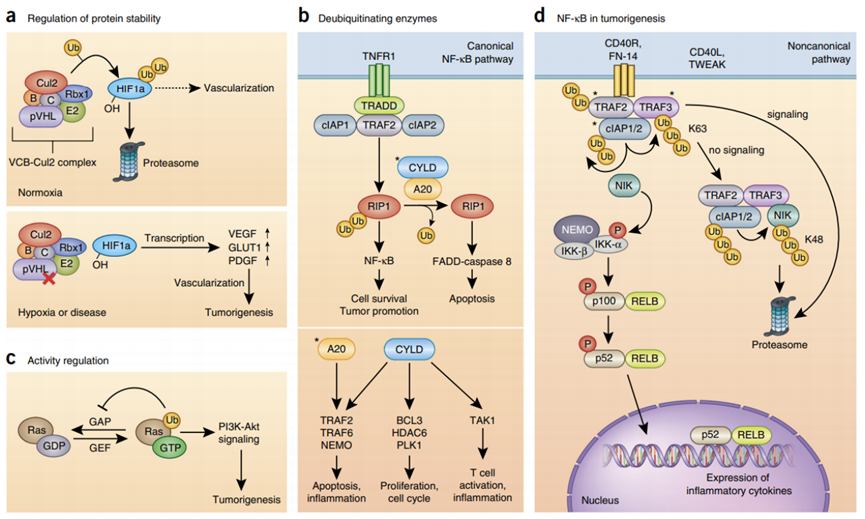
Ubiquitination is a crucial post-translational modification, with the ubiquitin-proteasome system being responsible for 80% to 85% of protein degradation in eukaryotic cells. Besides protein degradation, ubiquitination modification also plays a significant role in the regulation of various cellular processes, such as cell cycle, proliferation, apoptosis, differentiation, transcriptional regulation, gene expression, signaling, injury repair, and inflammatory immunity. This modification has been closely linked to the development of diseases such as tumors and cardiovascular disorders. As a significant breakthrough in biochemical research in recent years, ubiquitination has become a new target for the research and development of novel drugs.
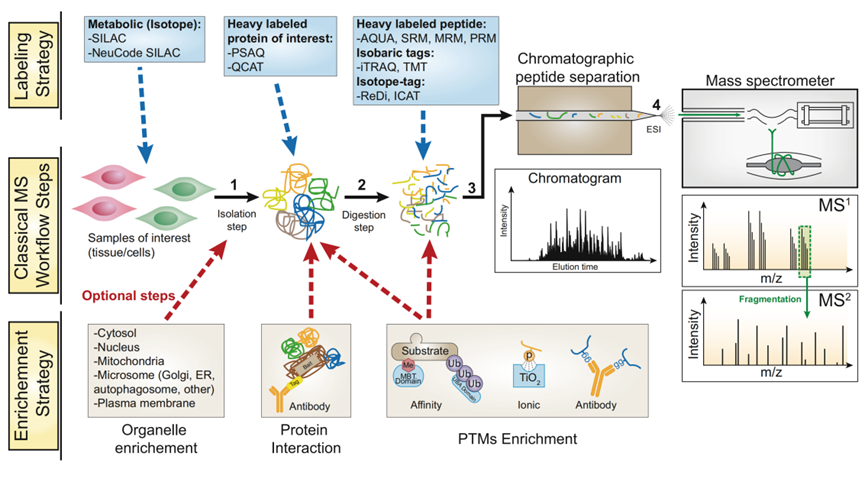
The protein sample is first subjected to enzymatic digestion to generate a peptide mixture. The resulting mixture is then fractionated using liquid chromatography to reduce sample complexity. Modified peptides are then enriched using high-quality antibodies specific to the desired modification, such as phosphorylation. The enriched peptides are then analyzed and quantified using liquid chromatography-tandem mass spectrometry.
Ubiquitination modification plays a crucial role in regulating various cellular processes, including cell cycle progression, proliferation, apoptosis, differentiation, transcriptional and signaling regulation, gene expression, injury repair, and inflammatory immunity. Dysregulated ubiquitination has been implicated in the pathogenesis of various diseases, such as tumors, cardiovascular disorders, and other diseases. Therefore, it has emerged as a significant research focus in biochemical research, offering potential new targets for drug development.
1.Study on physiological mechanism:Ubiquitin modification is involved in the regulation of almost all life activities, including signal transduction, transcription regulation, cell cycle, proliferation and apoptosis, DNA damage repair, inflammatory immunity and so on.
Nat Immunol: Integrative proteomics reveals an increase in non-degradative ubiquitylation in activated CD4(+) T cells.
2.Pathological study:Abnormal regulation of ubiquitin modification is often accompanied by diseases, such as cancer, cardiovascular disease, neurodegenerative disease, liver disease and so on.
Nat Commun: Proteome-wide analysis of USP14 substrates revealed its role in hepatosteatosis via stabilization of FASN.
3.Agriculture and Forestry:Ubiquitination modification is closely related to plant physiology. Many studies have proved that ubiquitination modification is involved in plant growth and development, abiotic stress, plant metabolism, biological stress and so on.
Plant Journal: Quantitative ubiquitylomics approach for characterizing the dynamic change and extensive modulation of ubiquitylation in rice seed germination.
Herdelberger J, et al., 2018, Proteomic frofiling of VCP substrates links VCP to K6-linked ubiquitylation and c-Myc function. EMBO Rep.
Interested in our services
Contact our experts to provide further information