Interested in our services
Contact our experts to provide further information



Succinylation is a post-translational modification in which a succinyl group is covalently attached to a lysine residue by enzymatic or other means, using a succinate donor.
Succinylation is a common post-translational modification in organisms, especially in many metabolic enzymes of central and intermediate metabolism. Succinylation modifications play an essential role in metabolic regulation, signal transduction, and many other biological processes. The modification has been shown to be closely related to various diseases, including inflammation, metabolic disorders, and tumors.
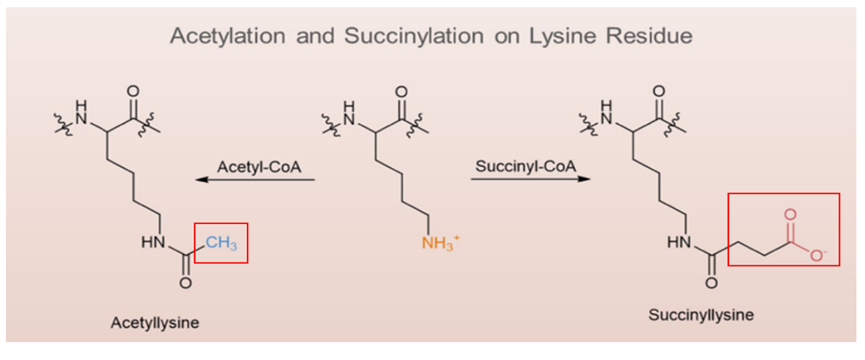
Compared to methylation and acetylation, succinylation modifications can induce greater changes in protein properties. This is because lysine residues that undergo succinylation gain two negative charges, resulting in a change of valency from +1 to -1, which is greater than the charge change caused by acetylation (+1 to 0) and monomethylation (no change). Additionally, succinylation introduces a larger chemical group, which can alter the structure and function of proteins more significantly.
The protein sample is first enzymatically digested into a peptide mixture, then fractionally separated the enzymatic peptide mixture using liquid chromatography to reduce sample complexity, then enriched the modified peptide by high-quality modified antibodies and biological materials, and finally loaded into liquid chromatography-tandem mass spectrometry for analysis and quantification.
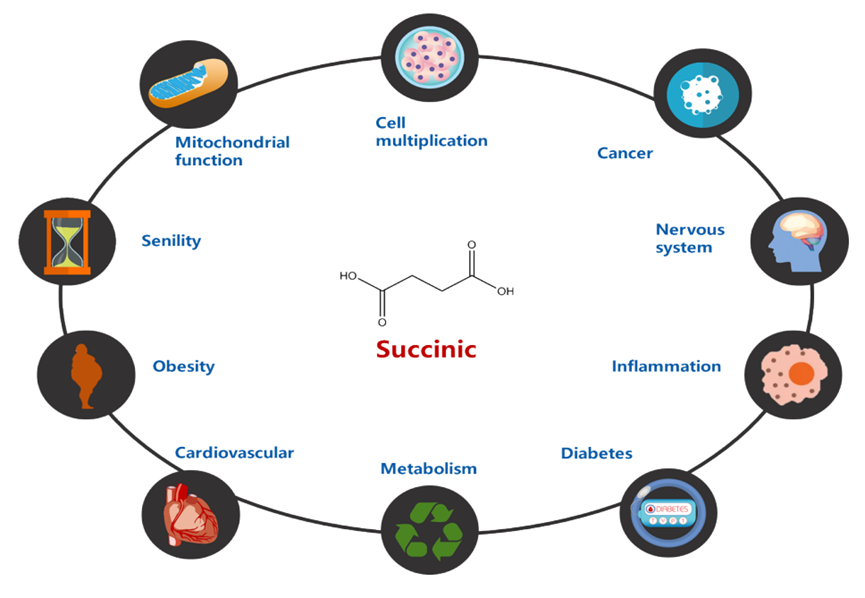
Succinic acid, which serves as the substrate for succinylation modification, is a metabolite involved in mitochondrial respiration. In addition to its role in energy metabolism, it has also been reported to participate in various physiological processes, such as stress response, inflammation, neural regulation, and immune regulation. Consequently, it can affect multiple diseases, including aging, obesity, tumorigenesis, cardiovascular disease, and neurodegenerative diseases.
Succinylation modification is a common occurrence in biological processes and is associated with diseases related to mitochondrial function, including mitochondrial metabolism and respiration, among others.
In addition to the biological processes related to succinic acid and mitochondrial function, succinylation modification is also involved in various other processes, such as the nervous system, inflammation, tumors, metabolism, and nucleosome regulation.
1. Metabolic regulation: Succinylation modification is a widespread regulator of cell metabolism, particularly in enzymes involved in mitochondrial energy metabolism, and is involved in the regulation of multiple metabolic signaling pathways, including the tricarboxylic acid cycle, amino acid metabolism, and fatty acid metabolism.
Cell Metabolism: SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks.
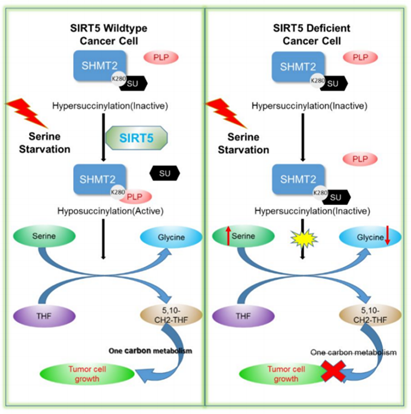
2.Tumor proliferation: Protein succinylation can modify the rate-limiting enzymes involved in metabolic pathways such as glycolysis, TCA cycle, and glutamate metabolism, thereby affecting various diseases, including tumors and inflammation.
Cancer Research: Oxidative stress-CBP axis modulates MOB1 acetylation and activates the Hippo signaling pathway.
3. Cardiovascular disease: succinylation modification plays a crucial role in regulating energy metabolism and oxidative stress, and it has been found to be closely associated with cardiovascular diseases, such as myocardial hypertrophy and atrial fibrillation.
PNAS: Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function
4. Plant stress: succinylation modification participates in glycolysis, TCA cycle and pentose phosphate pathway, and then regulates plants to cope with stress.
Plant Cell Environ: Oxidative stress-triggered interactions between the succinyl- and acetyl-proteomes of rice leaves.
Sadhukhan, et al., 2016, Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proceedings of the National Academy of Sciences.
Interested in our services
Contact our experts to provide further information