Interested in our services
Contact our experts to provide further information



Crotonylation is a novel type of acylation modification that was first identified by Professor Yingming Zhao's research team at the University of Chicago in 2011. This modification involves the addition of crotonyl groups to histone amino acid residues, primarily on lysine residues, and is known to play a crucial role in regulating gene expression and other important biological processes. In recognition of its significance, Cell Magazine selected crotonylation as the annual research highlight in the field of epigenetics.
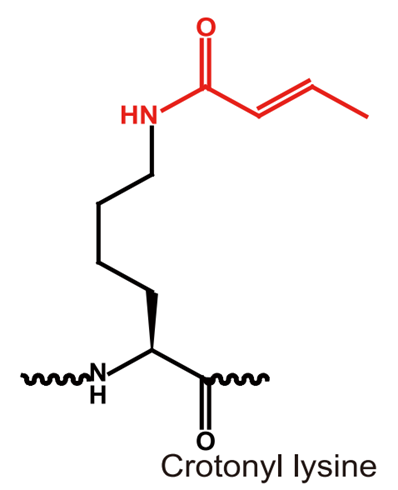
Crotonylation modifications are highly conserved throughout evolution and are known to preferentially bind to actively transcribed chromatin regions, playing a crucial role in regulating gene expression. Crotonylation modifications have been linked to reproductive regulation and have been shown to play a role in maintaining the activity of sex chromosome-associated genes during meiosis.
The protein sample is initially enzymatically digested into a mixture of peptides. Then, liquid chromatography is used to fractionate the peptide mixture to reduce the sample's complexity. Next, modified peptides are enriched using high-quality antibodies specific to the phosphorylated modification and biological materials. Finally, the enriched sample is analyzed and quantified using liquid chromatography-tandem mass spectrometry.
1.Crotonylation involves biological functions:
Gene expression and transcription
Reproductive regulation
Cell cycle
DNA damage response
Telomere maintenance and aging
Stem cell related
2.Crotonylation involves diseases:
Neurological disease
HIV
Kidney related diseases
Hypertrophic cardiomyopathy
Cancer related
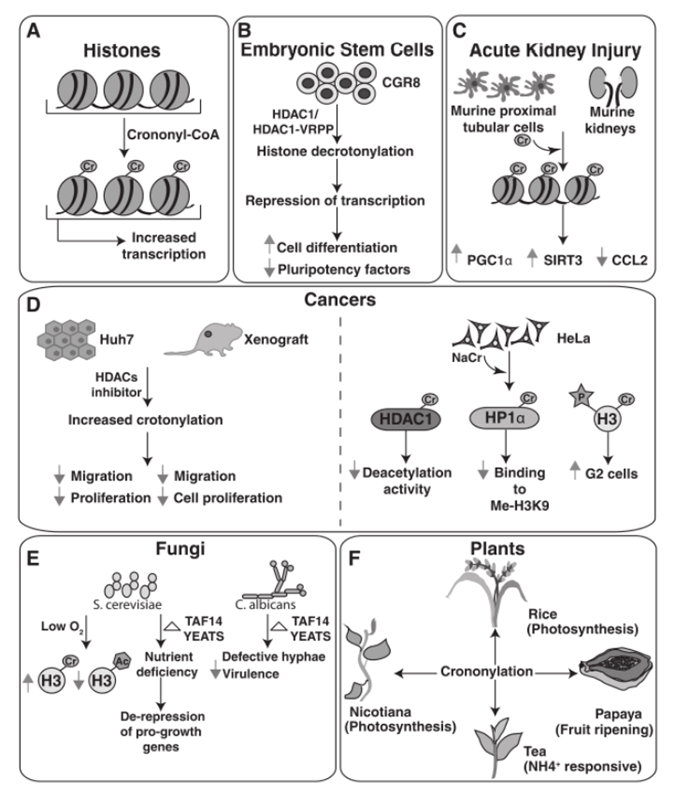
Proteomic analysis of crotonylation modification has been widely used in various research areas, including transcriptional regulation, cancer metabolism, DNA damage repair, stem cell differentiation, and reproductive regulation. Due to its versatility and importance, crotonylation modification proteomic analysis has become a major research focus in the field of life sciences.
1.Epigenetics
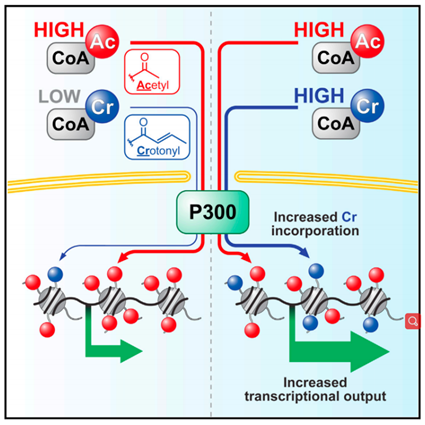
Molecular Cell: Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation
Coactivator p300 possesses both crotonyl transferase and acetyltransferase activities. The histone crotonylation catalyzed by p300 can directly stimulate transcription more than histone acetylation. The level of histone crotonylation is regulated by the concentration of crotonyl CoA in cells, which can change due to genetic and environmental factors. Local changes in histone acylation differences, i.e., acetylation and crotonylation, can impact gene expression.
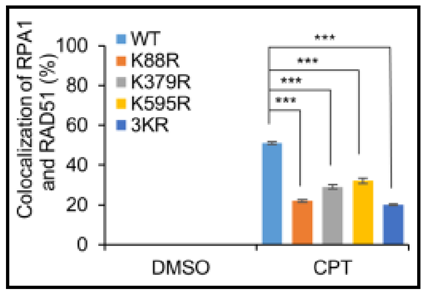
2.DNA damage repair
Science Advances: Global crotonylome reveals CDYL-regulated RPA1 crotonylation in homologous recombination-mediated DNA repair
Histomics data has revealed that rpa1 undergoes crotonylation modification at K88, K397, and K595 sites. Despite not interfering with its recruitment to DNA damage sites, the modification can alter rpa1's binding affinity with other homologous recombination factors such as Rad51, subsequently affecting rpa1's DNA damage repair function.
3.Reproductive development
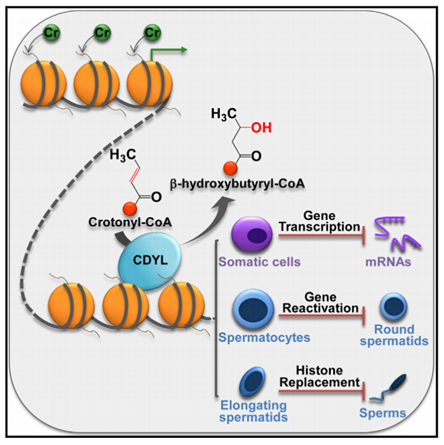
Molecular Cell: Chromodomain protein CDYL acts as a crotonyl-CoA hydratase to regulate histone crotonylation and spermatogenesis
CDYL is a protein that plays a role in the regulation of histone crotonylation levels by decreasing the substrate concentration. In mouse testis, CDYL and histone crotonylation exhibit mutually exclusive distribution, suggesting a negative regulatory relationship between them. Overexpression of CDYL in mice leads to a decrease in the level of histone crotonylation, which affects the reactivation of genes related to sex chromatin in the round spermatogenesis and the replacement of histones in the elongated spermatogenesis. This ultimately results in a reduction in sperm number and motility.
4.Tumor metabolism
Cell Research: Global profiling of crotonylation on non-histone proteins
Shumeng Liu, et al., 2017, Chromodomain protein CDYL acts as a crotonyl-CoA hydratase to regulate histone crotonylation and spermatogenesis. Molecular cell.
Interested in our services
Contact our experts to provide further information