Interested in our services
Contact our experts to provide further information



Malonylation is an evolutionarily conserved type of protein post-translational modification (PTM), which was first discovered on lysine in 2011. Its occurrence depends on the addition of malonyl groups to lysine by malonyl coenzyme A, changing its charge from +1 to -1. This change may disrupt the electrostatic interaction between lysine and other amino acids, alter the protein conformation, and even affect its binding to target proteins. Malonylation has been shown to exist in various metabolic pathways, such as fatty acid synthesis and oxidation, mitochondrial respiration, and glycolysis, which are closely related to type 2 diabetes and inflammation. As a result, malonylation has become an increasingly important area of research in recent years, as it may hold promise as a new target for the development of novel therapeutics.
1. Nature Communications:Malonylation of GAPDH is an inflammatory signal in macrophages.
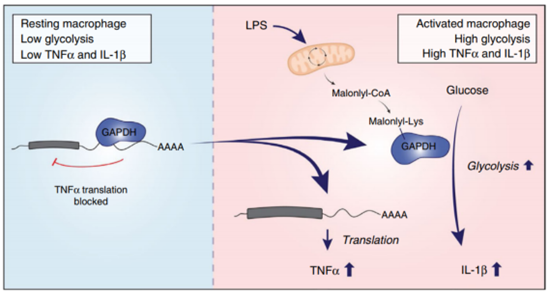
The study highlights the close relationship between macrophages' inflammatory response, metabolism, and malonylation modification, and provides insights into how macrophages regulate the expression of inflammatory-related factors through protein malonylation modification in vivo. Using quantitative omics analysis of mouse bone marrow-derived macrophages before and after lipopolysaccharide (LPS) treatment, the study found that malonylation modification was involved in various functions, including RNA regulation, signal transduction, and immune response. The study also identified that the K213 site on GAPDH was modified by malonylation. Normally, GAPDH binds to and inhibits the translation of several inflammation-related mRNAs, including TNFα. However, once GAPDH is modified by malonylation, it dissociates from the mRNA, promoting its translation and leading to increased TNFα production.
2. EMBO J: TFAM loss induces nuclear actin assembly upon mDia2 malonylation to promote liver cancer metastasis.
This study provides a novel link between mitochondria and the metastasis of liver cancer by revealing the mechanism of mitochondrial nuclear retrograde signaling. Specifically, the down-regulation of transcription factor A mitochondrial (TFAM) in liver cancer cells inhibits the mitochondrial tricarboxylic acid (TCA) cycle, leading to the accumulation of malonyl-CoA, which mediates malonylation modification and nuclear translocation of mdia2. This, in turn, promotes actin aggregation and the expression of multiple metastasis-related genes, ultimately resulting in the metastasis of liver cancer.

3. Molecular and Cellular Proteomics: Lysine malonylation is elevated in type 2 diabetic mouse models and enriched in metabolic associated proteins.
Researchers employed PAN antibodies modified for protein enrichment to identify and isolate liver tissue proteins from db/db mice with type 2 diabetes. They discovered that malonylation was significantly up-regulated in the liver tissue of the diabetic mice compared to wild-type littermates, whereas other modifications such as lysine acetylation, methylation, succinylation, butyrylation, propionylation, and crotonylation were only slightly up-regulated. Bioinformatics analysis indicated that malonylation-modified proteins played a critical role in glucose and fatty acid metabolism.
Interested in our services
Contact our experts to provide further information